Figures & data
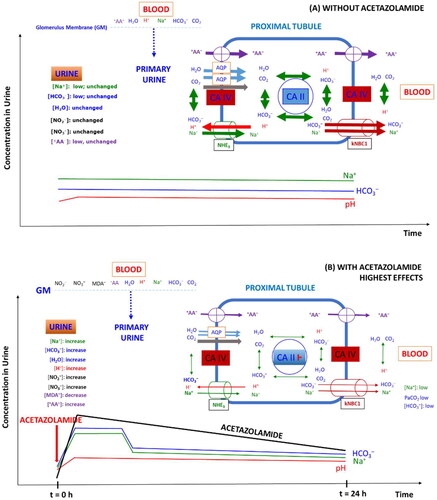
Figure 1. Published and archived articles in PubMed (https://pubmed.ncbi.nlm.nih.gov/) using the search term “carbonic anhydrase acetazolamide” 1952 to date (2023) and (date of search, 26 September 2023). As much as 3504 articles were published with a mean yearly publication rate of 49 articles. The number of articles using the term “carbonic anhydrase” amounts to 19,193. Inset shows the chemical structure of non-protonated AZM (N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide).
Figure 2. Time course of (A) the creatinine concentration in the urines samples collected in the study, of (B) the creatinine-corrected excretion rate of AZM (AZM), of (C) the creatinine-corrected excretion rates of nitrate and nitrite, and of (D) the urinary nitrate-to-urinary nitrite (UNOxR) in the respective urine samples without (blue) and with acidification (red) using 20 wt% acetic anhydride. Acidification of urine is required for the accurate measurement of nitriteCitation12, but not for other analytes including nitrate, creatinine, AZM and amino acids. AUC, area under the curve. AZM, acetazolamide.
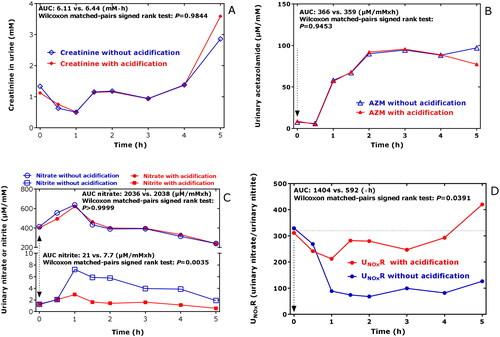
Table 1. Urinary creatinine-corrected excretion rates (µmol analyte/mmol creatinine) of nitrite, nitrate and malondialdehyde (acidified urine samples), amino acids and some of their metabolites (non-acidified urine samples) before (0 h) and until 5 h of AZM intake (a 250-mg tablet Acemit®; Medphano, Germany) by a healthy female volunteer.
Table 2. Results of the time series analysis (ARIMA) with respect to the AZM-induced changes in creatinine-corrected urinary excretion rate of the analytes alongside their chemical structures and electrical charge at physiological pH values of plasma and urine.
Table 3. Literature pharmacokinetic data of acetazolamide in humans.
Table 4. Literature pharmacodynamics data of acetazolamide in humans.
Figure 3. Simplified schematic of the role of carbonic anhydrase isoforms CA II (cytosolic) and CA IV (membrane-bound) and some transporters in the proximal tubule of the healthy kidney on the reabsorption of Na+, HCO3–, water, nitrite, nitrate, malondialdehyde (MDA) and zwitterionic amino acids (+AA-) under two different conditions: (A) Under physiological conditions, i.e. in the absence of AZM; (B) in the presence of AZM; AZM was taken orally at the time point zero as indicated by the vertical red coloured arrow; the time-course of the concentration of AZM in the urine are shown in addition to that of bicarbonate and sodium ions as well as of the urinary pH. AA, amino acid; AQP, aquaporin; GM, glomerular membrane; NHE3, sodium hydrogen exchanger; kNBC1, sodium bicarbonate transporter. It is assumed that CO2 is transferred by aquaporin and protein gas channels Citation98. The thickness of arrows indicate the status of the activity of carbonic anhydrase and the transporters. In case of ingestion of a sustained (retard) AZM, the maximal concentrations may be different and longer lasting Citation11–13. For more details, see the text.