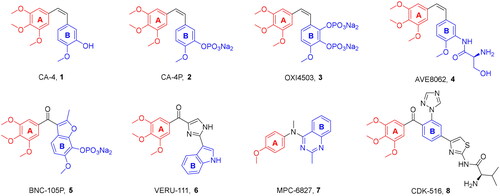
Figures & data
Scheme 1. Reagents and conditions (a) m-chloroperbenzoic acid, N2 atmosphere, rt., overnight; (b) HNO3/H2SO4, 60–90 °C, 1 h; (c) N,N-dimethylformamide dimethyl acetal, N,N-dimethylformamide, 120 °C, 4 h; (d) iron powder, acetic acid, 100 °C, 5 h; (e) 3,4,5-trimethoxyphenylboric acid, K2CO3, pyridine, Cu(OAc)2, 1,4-dioxane, N2 atmosphere, 85 °C, microwave (M.W), 30 min; (f) Substituted phenylboronic acid, Pd(PPh3)4, K2CO3, 1,4-dioxane/H2O, N2 atmosphere,125 °C, M.W., 26 min.
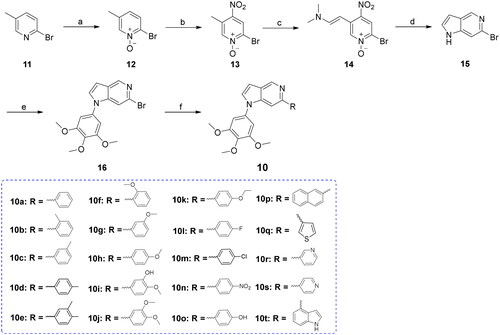
Table 1. Antiproliferative activity of all compounds.
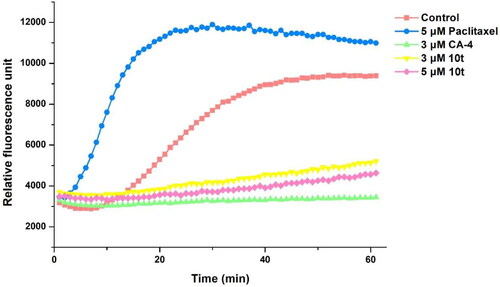
Figure 4. Effects of compound 10t (1 IC50) and CA-4 (1 IC50), on the cellular microtubule network and microtubule reassemble by immunofluorescence.
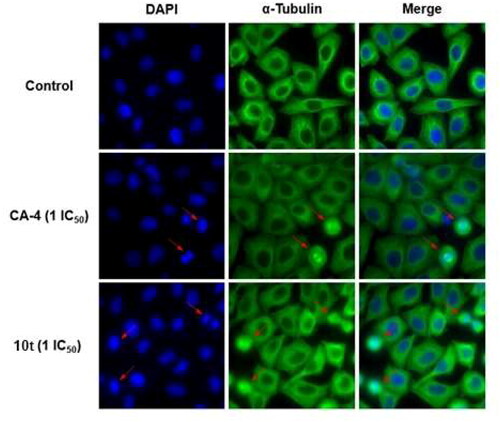
Figure 5. Effects of CA-4 and compound 10t on cell cycle. HeLa cells were treated with compound 10t (1 IC50, 2 IC50, and 3 IC50) for 24 h.
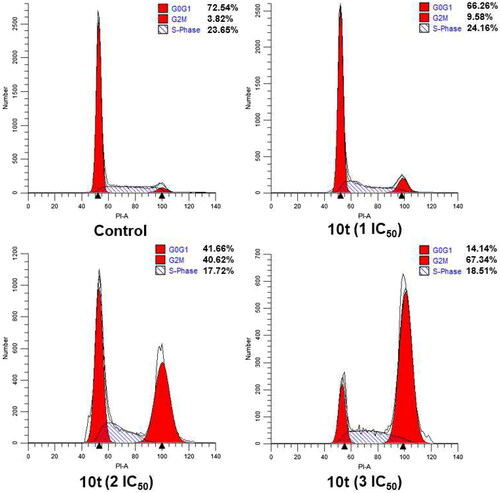
Figure 6. Analyses of apoptosis induction in Hela cells. Cells were harvested and stained with Annexin-V/PI for analysis after treatment with different concentrations of compound 10t (1 IC50, 2 IC50, and 3 IC50) and control for 48 h. The diverse cell stages were given as live (Q4), early apoptotic (Q3), late apoptotic (Q2), and necrotic cells (Q1).
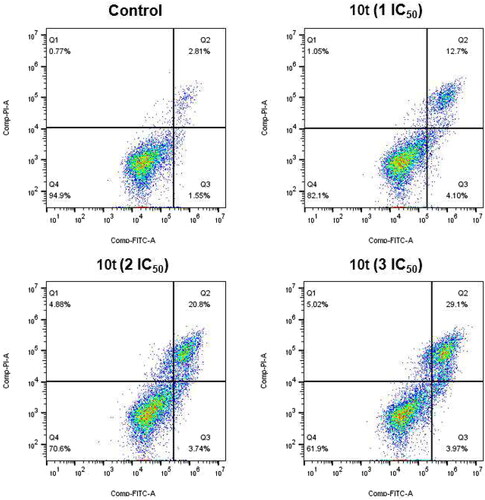
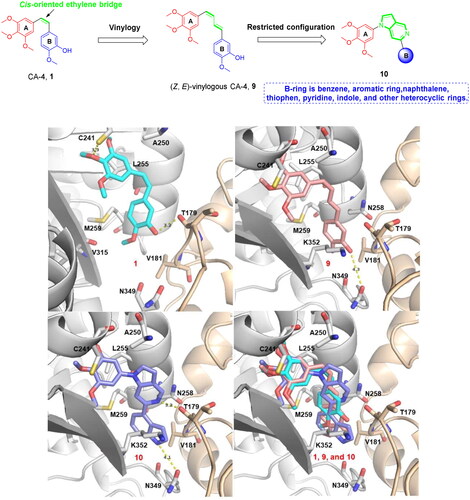
Figure 7. Proposed binding modes for 10t (C) in comparison with CA-4 (A) and 7 (B) at the colchicine-site. Carbon atoms are shown in cyan for CA-4, in salmon for 7, and in pale purple for 10t. The residues from the α-tubulin chain are shown in pale yellow, whereas residues from β-tubulin are coloured in gray.
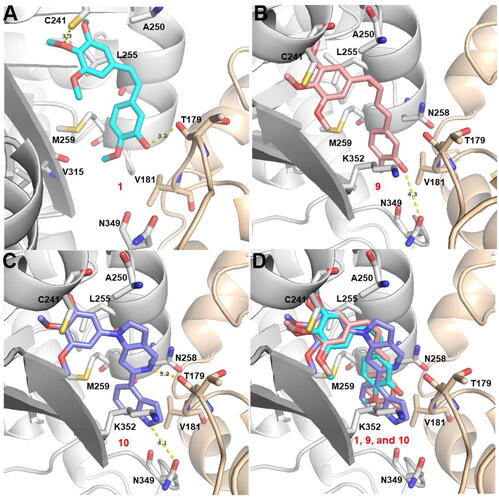
Table 2. Prediction of physicochemical properties of CA-4, 9i, and 9pTable Footnotea.