Figures & data
Table 1. In vitro COX-1, COX-2, and 5-LOX enzyme inhibitory activities, aIC50 values and bSI of the tested compounds.
 .
.
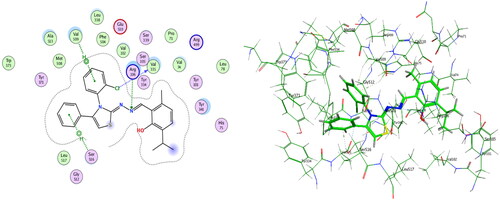
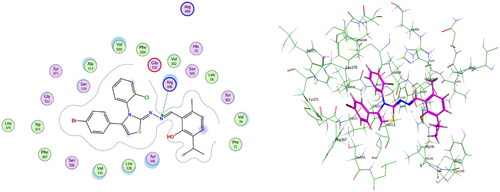
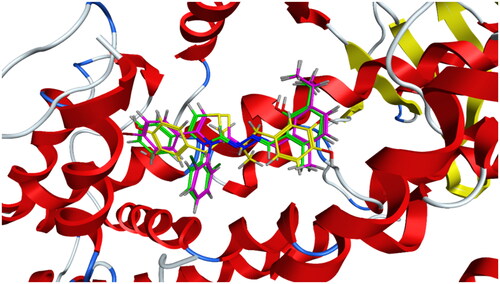
Figure 5. Left: co-crystallized celecoxib, ligand-enzyme interaction (2D). Right: Overlay of co-crystallized celecoxib (yellow) and docked celecoxib (cyan) with RMSD = 0.422 (3D) inside COX-2 active site.
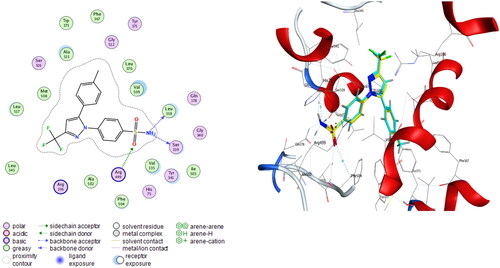
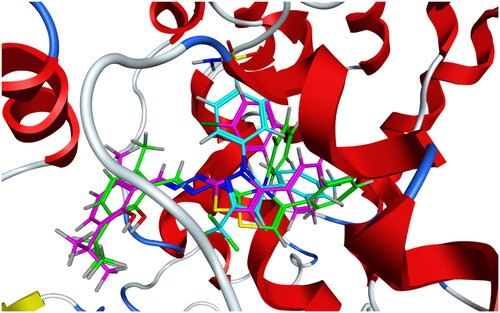
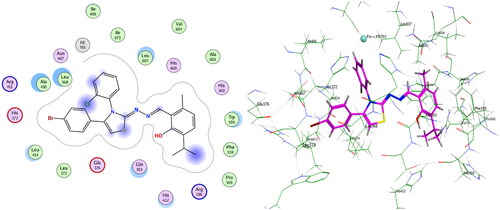
Table 2. Induced fit docking results of the active compounds inside COX-2 active site.
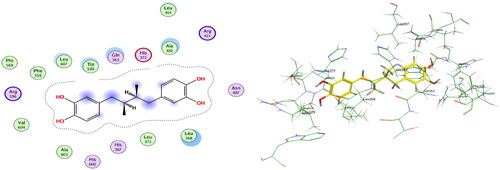
Figure 10. Overlay of NDGA (cyan) and docked NDGA (yellow) inside 5-LOX active site with RMSD = 1.2.
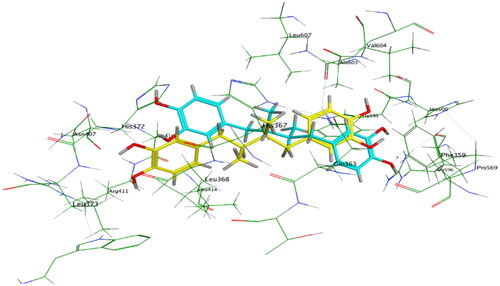
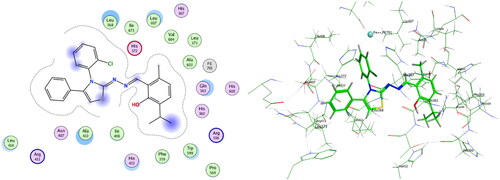
Table 3. Induced fit docking results of the active compounds in 5-LOX active site.
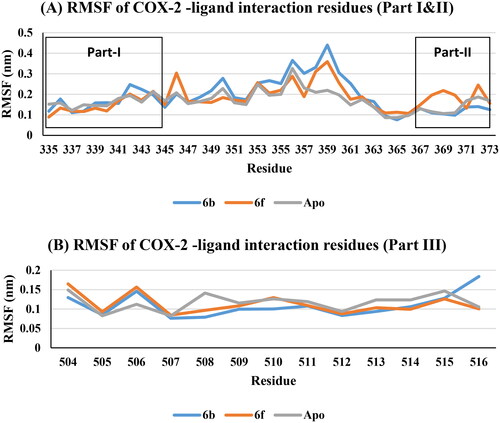
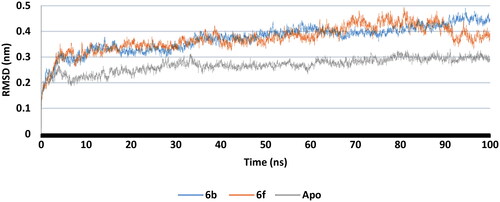
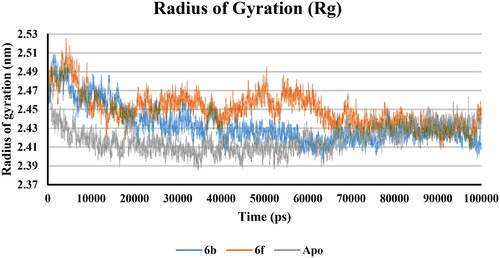
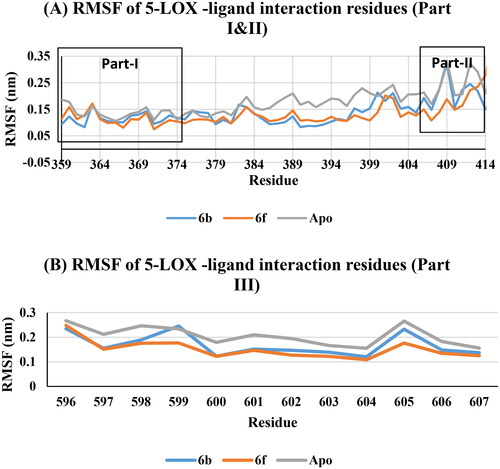
Figure 15. RMSF of COX-2 -ligand interaction residues, Apo (Grey), 6b (Blue), and 6f (red): (A) Part I and II and (B) Part III.