Figures & data
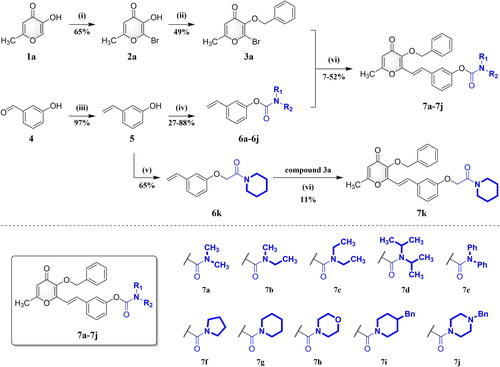
Scheme 1. Synthesis of compounds 7a–7k. Reagents and conditions: (i) NBS, NH4OAc, CH3CN, RT; (ii) BnBr, K2CO3, CH3CN, 75 °C; (iii) CH3Ph3P-Br+, NaH, THF, N2, 80 °C; (iv) R1R2NCOCl, 4-DMAP, K2CO3, CH3CN, N2, 75 °C; (v) 2-chloro-1-(piperidin-1-yl)ethan-1-one, 4-DMAP, K2CO3, CH3CN, N2, 75 °C; (vi) Pd(OAc)2, TEA, DMF, N2, 100 °C.
Scheme 2. Synthesis of compounds 7l–7s. Reagents and conditions: (A) (i) NBS, NH4OAc, CH3CN, RT; (ii) BnBr, K2CO3, CH3CN, 75 °C; (iii) 2-(bromomethyl)naphthalene, K2CO3, CH3CN, 75 °C; (iv) 4-(bromomethyl)-1,1’-biphenyl, K2CO3, CH3CN, 75 °C; (v) CH3I, K2CO3, DMF, RT; (vi) Pd(OAc)2, TEA, DMF, N2, 100 °C. (B) (i) PdCl2(dppf), K2CO3, THF: H2O (9:1, v/v), N2, 75 °C; (ii) Piperidine-1-carbonyl chloride, 4-DMAP, K2CO3, CH3CN, N2, 75 °C. (C) (i) H2, 10% Pt/C, THF, 50 °C. (D) X ray of compound 7p (CCDC Number 2299602).
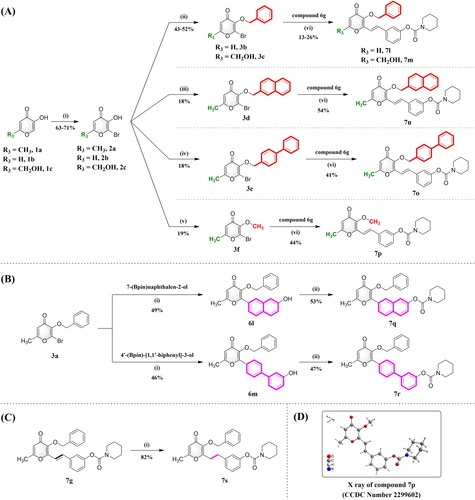
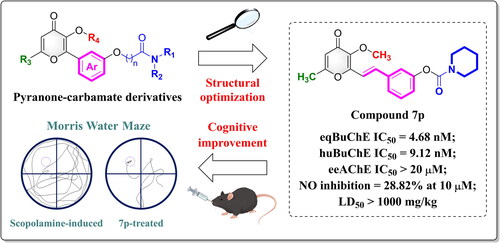
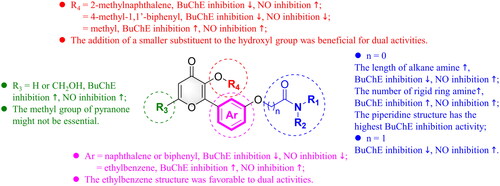
Table 1. In vitro eeAChE, eqBuChE, and huBuChE inhibitory assay results (IC50 values)Table Footnotea of compounds 7a–7k.
Table 2. In vitro eeAChE, eqBuChE, and huBuChE inhibitory assay results (IC50 values)Table Footnotea of compounds 7l–7s.
Figure 1. Inhibitory activity of hydrocortisone and compounds 7a–7s on nitric oxide production. The data are expressed as the mean ± SEM of at least three experiments and analysed with a one-way ANOVA followed by Dunnett’s post hoc analysis. ####p < 0.0001 compared to the control group; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to the LPS model group. LPS: lipopolysaccharide; HC: Hydrocortisone.
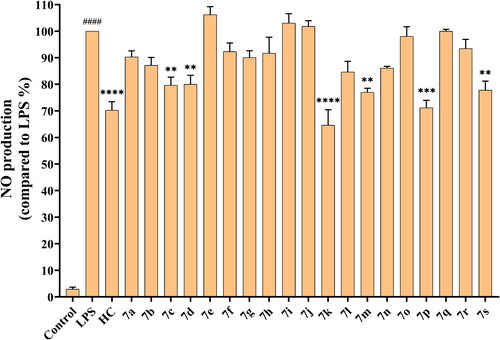
Figure 3. BuChE inhibition kinetics of compound 7p. (A) Dixon plot of compound 7p; (B) Cornish-Bowden plot of compound 7p.
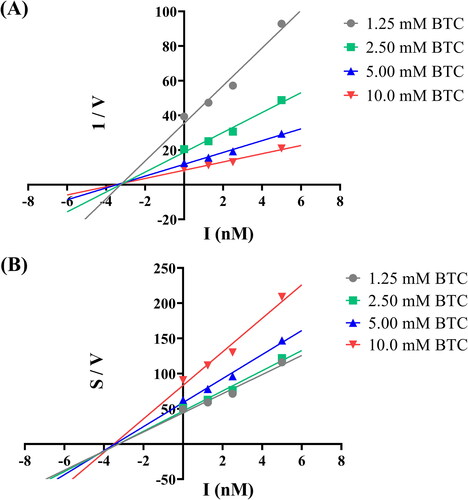
Figure 4. Docking studies of compound 7p. (A) The binding pocket of compound 7p with BuChE by the surface representation; (B) The interactions of compound 7p with BuChE (PDB code: 1P0I); (C) The binding pocket of compound 7p with AChE by the surface representation; (D) The interactions of compound 7p with AChE (PDB code: 4EY7).
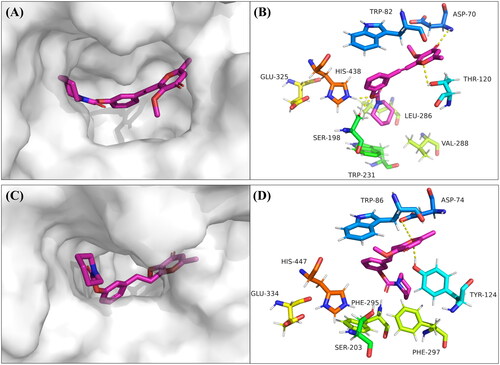
Table 3. Permeability Pe of six commercial drugs and compound 7p in the PAMPA-BBB assay.
Figure 5. Toxicity evaluation of compound 7p. (A) The trends of body weight changes in control and 7p-treated group (14 d). (B) The histomorphological appearance of heart, liver, spleen, and brain between control and 7p-treated group (40 ×, scale at 50 μm).
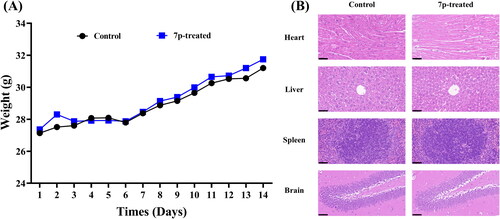
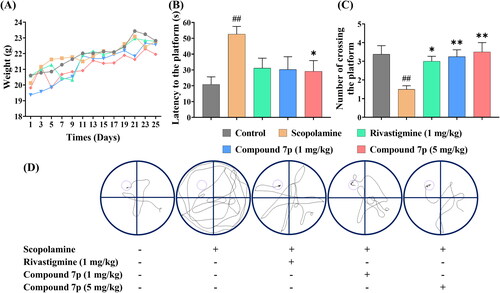
Figure 6. The effects of Rivastigmine and compound 7p on scopolamine-induced cognitive impairment in the Morris water maze test. (A) The daily weight of mice in different groups during the entire period. (B) The escape latency to the platform on the fifth day of training trials. (C) The number of crossing the platform on the last day without platform. (D) The representative tracks of mice on the fifth day of training trials. The data are expressed as the mean ± SEM and analysed with a one-way ANOVA followed by Dunnett’s post hoc analysis. ##p < 0.01 compared to the control group; *p < 0.05, **p < 0.01 compared to the scopolamine model group.