Figures & data
Figure 2. (A) docking pose of compound 11 in complex with Mpro (PBD ID 5R80) (left) and the 2D interaction map (right) showing the fundamental interactions of certain moieties in 11 with the protein; (B) The suggested modification aiming to improve its binding with the protein and enhance its anti-Covid activity.
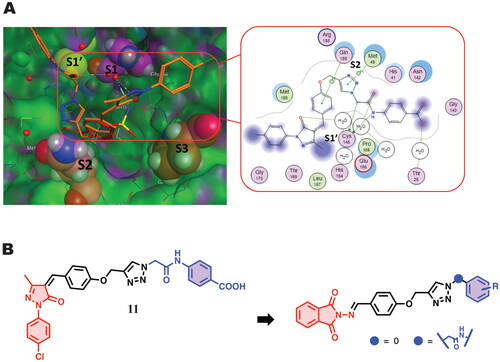
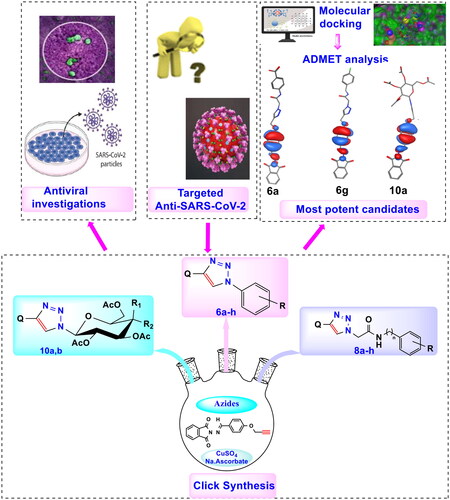
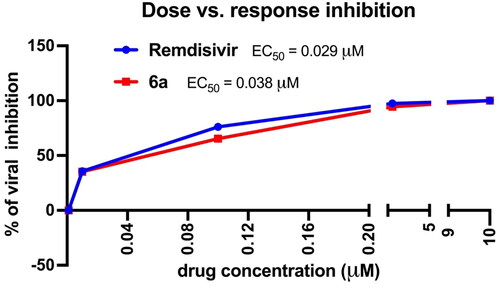
Table 1. Antiviral activity of novel phthalimide-based triazole inhibitors by propagation in Vero E6 cells.
Table 2. Inhibitory data of selected derivatives against SARS-CoV-2 Mpro.
Figure 4. Docked poses of Mpro protease (PDB ID: 5R80) complexed with Z18197050 and 6 g compound with 2D interaction map for 6 g (right) and 3D interaction map of both aligned compounds (left).
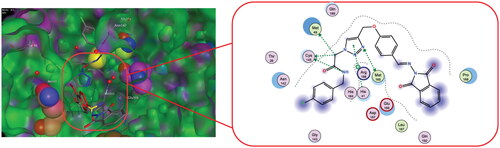
Table 3. Calculation of certain properties of compounds 6f, 6i, and 10a in comparison with reported drugs as predicted by OSIRIS Property Explorer and Molinspiration tools.
Table 4. Molecular mechanics parameters and molecular orbital spatial distribution and localisation for the HOMO-LUMO of representative compounds, 6a, 6 g, and 10a.
Supplemental Material
Download PDF (1.5 MB)Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.