Figures & data
Figure 1. Study design
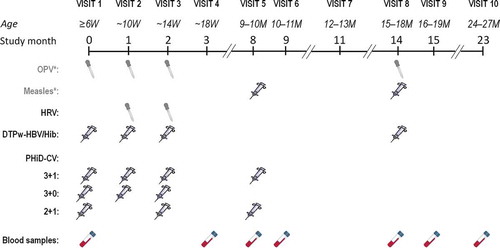
Table 1. Demographic characteristics (total vaccinated cohort)
Figure 2. Participant Flow Diagram
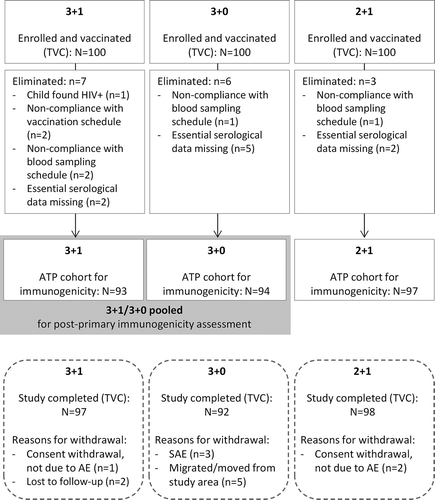
Table 2. Immunogenicity assessed by GSK 22F ELISA for antipneumococcal antibodies and anti-protein D ELISA (ATP cohort for immunogenicity)
Table 3. Functional immune response assessed by opsonophagocytic activity assay (ATP cohort for immunogenicity)
Figure 3. Kinetics of anti-pneumococcal antibody GMCs (ATP cohort for immunogenicity)
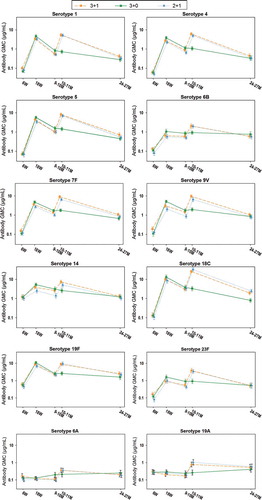
Figure 4. Kinetics of anti-pneumococcal OPA GMTs (ATP cohort for immunogenicity)
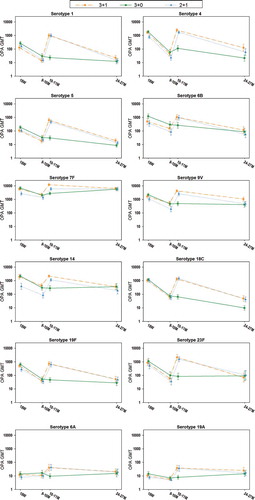
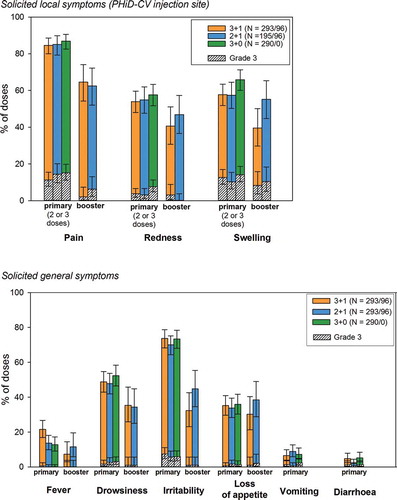
Figure 5. Solicited local and general symptoms (overall per dose; total vaccinated cohort)