Figures & data
Figure 1. Phylogenetic tree of the M. tuberculosis complex and depiction of M. tuberculosis, BCG and MTBVAC. The tree depicts the regions of difference (RDs) deleted between M. tuberculosis and M. bovis in the process of evolution with their host. BCG has lost additional RDs that have led to attenuation. The number of epitopes of some RD regions is also indicated. MTBVAC is represented to have the antigenic repertoire of M. tuberculosis absent in BCG, expected to confer protection against disease similar to latent M. tuberculosis infection in humans [Citation47], and at the same time presenting attenuation profile that is comparable to BCG
![Figure 1. Phylogenetic tree of the M. tuberculosis complex and depiction of M. tuberculosis, BCG and MTBVAC. The tree depicts the regions of difference (RDs) deleted between M. tuberculosis and M. bovis in the process of evolution with their host. BCG has lost additional RDs that have led to attenuation. The number of epitopes of some RD regions is also indicated. MTBVAC is represented to have the antigenic repertoire of M. tuberculosis absent in BCG, expected to confer protection against disease similar to latent M. tuberculosis infection in humans [Citation47], and at the same time presenting attenuation profile that is comparable to BCG](/cms/asset/336cc44a-616c-43cd-9379-9adaff166c74/ierv_a_1324303_f0001_c.jpg)
Figure 2. MTBVAC and SO2 genetic and phenotypic profile. Figure depicts the marked phoP mutation by insertion of a Kanamycin resistance cassette (Kmr) insertion and spontaneous loss in the virulence-associated phthiocerol dimycocersate (PDIM) lipids in SO2 and the stable unmarked genetic deletions in phoP and fadD26 in MTBVAC. Since MTBVAC was constructed using the SO2 background, MTBVAC also has the spontaneous loss in PDIM present in SO2 and deletion in fadD26 ensuring a genetically stable PDIM deficient phenotype
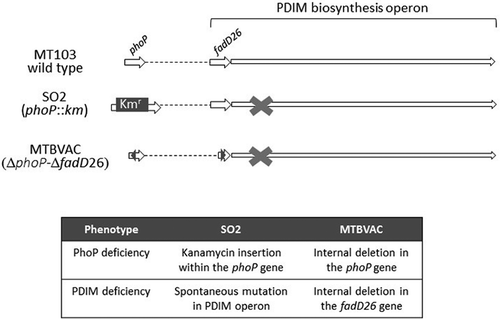
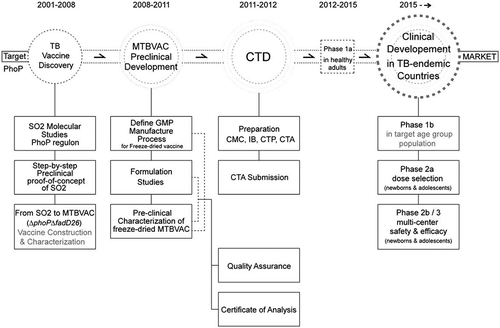
Figure 3. Development of MTBVAC from discovery to clinical development. The figure represents the chronological order of the steps in the discovery of phoP, construction and characterization of SO2 and MTBVAC, and the GMP, preclinical and clinical development of final product MTBVAC freeze-dried vaccine. Meaning of abbreviations: CTP – Clinical Trial Protocol; CMC – Chemistry, Manufacturing, and Controls; IB – Investigator’s Brochure; CTP – Clinical Trial Protocol; CTA – Clinical Trial Application