Figures & data
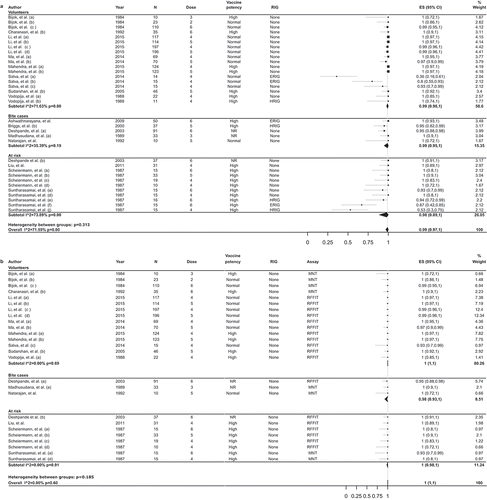
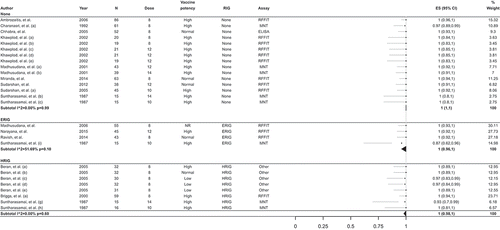
Table 1. RVNA GMTs from studies comparing IM and ID administration of PCECV.
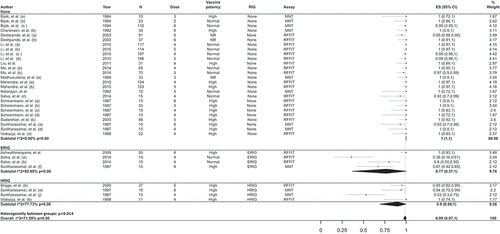
Table 2. Overview of the estimates for the percentage of participants with RVNA titers ≥0.5 IU/mL following vaccination with PCEV by IM and ID administration in interventional studies.
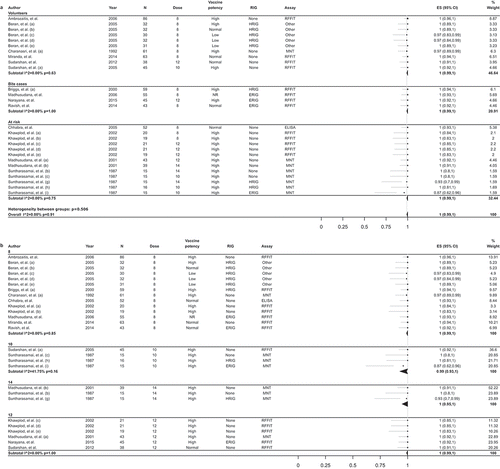
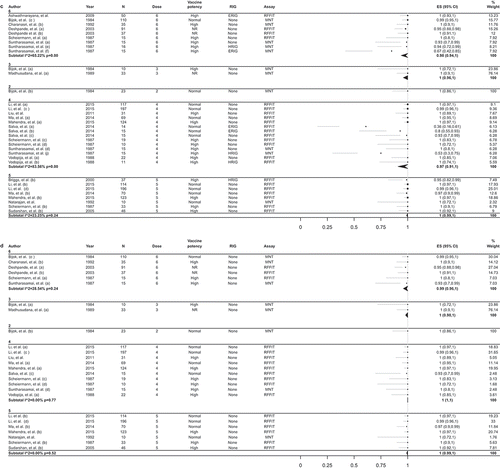
Table 3. Adverse reactions after PCECV by IM administration, reported in interventional studies.
Table 4. Adverse reactions after PCECV by IM administration reported in observational studies.
Table 5. Adverse reactions after PCECV by ID administration reported in interventional studies.