Figures & data
Figure 1. Three levels of protection elicited by PCVs against disease caused by VT pneumococci.
(1) Acquisition of circulating neutralizing antibodies (i.e. serotype-specific immunoglobulin G). (2) Prevention of VT NP acquisition following exposure to the pathogen. (3) Prevention of person-to-person transmission of VT pneumococci. Levels 1 and 2 represent protection of the vaccinated individual, often referred to as direct protection. Level 3 is referred to as indirect or herd protection. NP = nasopharyngeal; PCV = pneumococcal conjugate vaccine; VT = vaccine serotype.
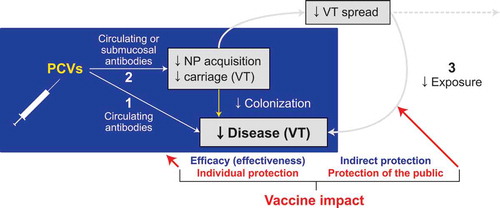
Figure 2. Differences in inferiority observed when measuring the proportion of children achieving concentrations above threshold vs geometric mean antibody concentrations: A comparison between PCV10 and PCV7.
(a) After the third primary vaccine dose, PCV10 was noninferior to PCV7 for most serotypes using the proportion of subjects achieving a titer of ≥0.20 µg/mL response; the only exceptions were serotypes 6B and 23F. (b) PCV10 was inferior to PCV7 for most serotypes when measuring the geometric mean concentrations of anti-polysaccharide immunoglobulin G; the only exception was serotype 19F. Solid bars, data from Wysocki J, et al. Pediatr Infect Dis J 2009 [Citation34]. Hatched bars, data from Vesikari T, et al. Pediatr Infect Dis J 2009 [Citation35]. ELISA = enzyme-linked immunosorbent assay; PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV.
![Figure 2. Differences in inferiority observed when measuring the proportion of children achieving concentrations above threshold vs geometric mean antibody concentrations: A comparison between PCV10 and PCV7.(a) After the third primary vaccine dose, PCV10 was noninferior to PCV7 for most serotypes using the proportion of subjects achieving a titer of ≥0.20 µg/mL response; the only exceptions were serotypes 6B and 23F. (b) PCV10 was inferior to PCV7 for most serotypes when measuring the geometric mean concentrations of anti-polysaccharide immunoglobulin G; the only exception was serotype 19F. Solid bars, data from Wysocki J, et al. Pediatr Infect Dis J 2009 [Citation34]. Hatched bars, data from Vesikari T, et al. Pediatr Infect Dis J 2009 [Citation35]. ELISA = enzyme-linked immunosorbent assay; PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV.](/cms/asset/d0b72fdd-b319-433d-af81-cd83c84f5409/ierv_a_1627207_f0002_c.jpg)
Figure 3. Carriage studies examining the efficacy of CRM197-conjugated vaccines (i.e. PCV7/PCV13) and protein-D conjugated vaccines (i.e. PCV10/PCV11).
(a) All published studies in which PCV was administered as three infant doses, and carriage was measured around 12 months of age, before the toddler dose. (b) All published studies in which PCVs were administered at either two or three doses during infancy, and a booster was administered at around 12 months of age. The age when carriage was measured appears on the y-axis. Several age points were often used for the same study. The circled endpoints are for studies in which children received two doses in infancy. For all others, children received three infant doses. Each circle represents the endpoint efficacy of the vaccine measured against placebo or control vaccine; error bars represent CIs. Adapted from Fleming-Dutra KE, et al. Pediatr Infect Dis J 2014 [Citation55]. Studies on y-axes correspond to: (a) Prymula R, et al. Vaccine 2009 [Citation57]; (b) GlaxoSmithKline COMPAS Study 109563 (10PN-PD-DIT-028) [Citation61]; (c) Vesikari T. European Congress of Clinical Microbiology and Infectious Diseases 2013, Berlin, Germany [Citation56]; (d) Cheung YB, et al. Pediatr Infect Dis J 2009 [Citation58]; (e) Russell FM, et al. Clin Vaccine Immunol 2010 [Citation48]; (f) Dagan R, et al. Clin Infect Dis 2013 [Citation42]; (g) O’Brien KL, et al. J Infect Dis 2007 [Citation59]; (h) Prymula R, et al. Vaccine 2011 [Citation62]; and (i) van Gils EJ, et al. JAMA 2009 [Citation60]. *Data are for the additional six serotypes in PCV13 plus 6C combined. PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV9 = 9-valent PCV; PCV10 = 10-valent PCV; PCV11 = 11-valent PCV; PCV13 = 13-valent PCV; VT = vaccine serotype.
![Figure 3. Carriage studies examining the efficacy of CRM197-conjugated vaccines (i.e. PCV7/PCV13) and protein-D conjugated vaccines (i.e. PCV10/PCV11).(a) All published studies in which PCV was administered as three infant doses, and carriage was measured around 12 months of age, before the toddler dose. (b) All published studies in which PCVs were administered at either two or three doses during infancy, and a booster was administered at around 12 months of age. The age when carriage was measured appears on the y-axis. Several age points were often used for the same study. The circled endpoints are for studies in which children received two doses in infancy. For all others, children received three infant doses. Each circle represents the endpoint efficacy of the vaccine measured against placebo or control vaccine; error bars represent CIs. Adapted from Fleming-Dutra KE, et al. Pediatr Infect Dis J 2014 [Citation55]. Studies on y-axes correspond to: (a) Prymula R, et al. Vaccine 2009 [Citation57]; (b) GlaxoSmithKline COMPAS Study 109563 (10PN-PD-DIT-028) [Citation61]; (c) Vesikari T. European Congress of Clinical Microbiology and Infectious Diseases 2013, Berlin, Germany [Citation56]; (d) Cheung YB, et al. Pediatr Infect Dis J 2009 [Citation58]; (e) Russell FM, et al. Clin Vaccine Immunol 2010 [Citation48]; (f) Dagan R, et al. Clin Infect Dis 2013 [Citation42]; (g) O’Brien KL, et al. J Infect Dis 2007 [Citation59]; (h) Prymula R, et al. Vaccine 2011 [Citation62]; and (i) van Gils EJ, et al. JAMA 2009 [Citation60]. *Data are for the additional six serotypes in PCV13 plus 6C combined. PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV9 = 9-valent PCV; PCV10 = 10-valent PCV; PCV11 = 11-valent PCV; PCV13 = 13-valent PCV; VT = vaccine serotype.](/cms/asset/662b9aa2-2f63-4a2f-a0b9-7c9f85ac7995/ierv_a_1627207_f0003_c.jpg)
Table 1. Immunogenicity of serotypes 19A and 6A using OPA assays from clinical studies comparing PCV7, PCV10, and PCV13.
Figure 4. Cross-protection induced by cross-reactive serotype antigens does not provide a similar level of protection to that elicited by the specific serotype antigen against serotype 19A carriage: results of two double-blind studies.
(a) Results of a double-blind study comparing PCV13 and PCV7 for 19A carriage in Israel. Adapted from Dagan R, et al. Clin Infect Dis 2013 [Citation42]. (b) Results of a study comparing the efficacy of PCV10 and PCV7 on 19A carriage in the Netherlands. Adapted from van den Bergh MR, et al. Clin Infect Dis 2013 [Citation83]. PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV; PCV13 = 13-valent PCV.
![Figure 4. Cross-protection induced by cross-reactive serotype antigens does not provide a similar level of protection to that elicited by the specific serotype antigen against serotype 19A carriage: results of two double-blind studies.(a) Results of a double-blind study comparing PCV13 and PCV7 for 19A carriage in Israel. Adapted from Dagan R, et al. Clin Infect Dis 2013 [Citation42]. (b) Results of a study comparing the efficacy of PCV10 and PCV7 on 19A carriage in the Netherlands. Adapted from van den Bergh MR, et al. Clin Infect Dis 2013 [Citation83]. PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV; PCV13 = 13-valent PCV.](/cms/asset/80066f20-042b-41ea-8654-532ddfa99db1/ierv_a_1627207_f0004_c.jpg)
Table 2. Postlicensure PCV10 and PCV13 studies assessing the effect of vaccination on serotype 19A, 6A, and 6C carriage.
Table 3. Impact of PCV10 and PCV13 against IPD caused by serotypes 19A, 6A, and 6C across all ages in countries with robust surveillance.
Figure 5. Differential impact on serotype 19A IPD post-PCV10 vs post-PCV13 implementation in six countries: three countries post-PCV13 introduction (Israel, United States, United Kingdom) and three countries post-PCV10 introduction (Chile, New Zealand, Finland).
(a) Number of cases of serotype 19A IPD in children <5 years of age between 2004 and 2016. (b) Number of cases of serotype 19A IPD in individuals ≥5 years of age between 2004 and 2016. Vertical dashed lines denote the implementation year of PCV7, PCV10, or PCV13. Vaccination with PCV10 was conducted in Chile and Finland per 3 + 1 and 2 + 1 schedules, respectively. Data are adapted from (a) Israel National Surveillance. Personal communication to Ron Dagan; (b) Moore MR, et al. Lancet Infect Dis 2015 [Citation134]; (c) S. Ladhani, Pediatric Infectious Disease, Public Health England, London, UK, personal communication, 12/30/2017; (d) Pan American Health Organization. SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables do Neumonias y Meningitis Bacterianas) [Citation125]; (e) Public Health Surveillance. Information for New Zealand Public Health Action. Invasive Pneumococcal Disease Reports [Citation118]; and (f) National Institute for Health and Welfare. Incidence of Invasive Pneumococcal Disease in Finland [Citation126]. *Number of cases (scale) per country differs. †Threshold for Chile is <2 years and ≥2 years of age. §Surveillance years may span January–December or July–June. IPD = invasive pneumococcal disease; PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV; PCV13 = 13-valent PCV.
![Figure 5. Differential impact on serotype 19A IPD post-PCV10 vs post-PCV13 implementation in six countries: three countries post-PCV13 introduction (Israel, United States, United Kingdom) and three countries post-PCV10 introduction (Chile, New Zealand, Finland).(a) Number of cases of serotype 19A IPD in children <5 years of age between 2004 and 2016. (b) Number of cases of serotype 19A IPD in individuals ≥5 years of age between 2004 and 2016. Vertical dashed lines denote the implementation year of PCV7, PCV10, or PCV13. Vaccination with PCV10 was conducted in Chile and Finland per 3 + 1 and 2 + 1 schedules, respectively. Data are adapted from (a) Israel National Surveillance. Personal communication to Ron Dagan; (b) Moore MR, et al. Lancet Infect Dis 2015 [Citation134]; (c) S. Ladhani, Pediatric Infectious Disease, Public Health England, London, UK, personal communication, 12/30/2017; (d) Pan American Health Organization. SIREVA II (Sistema de Redes de Vigilancia de los Agentes Responsables do Neumonias y Meningitis Bacterianas) [Citation125]; (e) Public Health Surveillance. Information for New Zealand Public Health Action. Invasive Pneumococcal Disease Reports [Citation118]; and (f) National Institute for Health and Welfare. Incidence of Invasive Pneumococcal Disease in Finland [Citation126]. *Number of cases (scale) per country differs. †Threshold for Chile is <2 years and ≥2 years of age. §Surveillance years may span January–December or July–June. IPD = invasive pneumococcal disease; PCV = pneumococcal conjugate vaccine; PCV7 = 7-valent PCV; PCV10 = 10-valent PCV; PCV13 = 13-valent PCV.](/cms/asset/ca58eef4-f45e-4989-883f-d2580a9b10fb/ierv_a_1627207_f0005_c.jpg)
