Figures & data
Figure 2. Selection of publications in the systematic review. AOM, acute otitis media; IPD, invasive pneumococcal disease; n, number of records; PCV(9/13), (9/13-valent) pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.
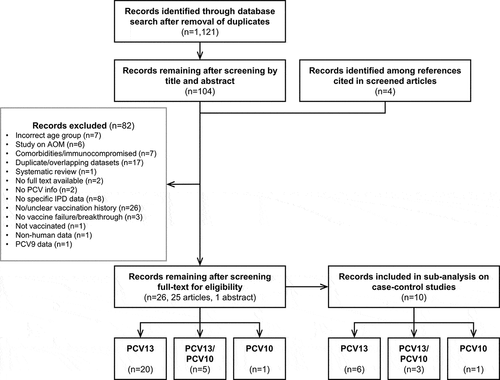
Table 1. Characteristics of included studies
Table 2. Breakthrough IPD cases and vaccine failures identified in the included studies
Figure 3. Vaccine failures and breakthrough IPD cases after PCV13 or PCV10, reported for each serotype in the included studies, shown as proportions of the total vaccine failures or breakthrough cases across serotypes. Panel a shows vaccine failures, and breakthrough cases after receipt of ≥1 dose or ≥2 doses of PCV13 or PCV10. Panel b shows breakthrough cases after receipt of exactly 1, 2 or 3 doses of PCV13 or PCV10. IPD, invasive pneumococcal disease; N, total number of reported vaccine failures or breakthrough cases after the indicated number of doses, including only cases with serotype information available; PCV13, 13-valent pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.
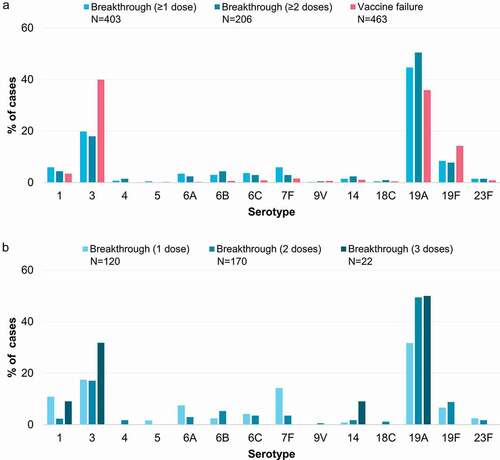
Figure 4. Vaccine failures after receipt of a 2+1, 3+0 or 3+1 PCV13 or PCV10 schedule reported for each serotype in the included studies, shown as proportions of the total vaccine failures across serotypes. 2+1, schedule consisting of 2 primary doses and a booster; 3+0, schedule consisting of 3 primary doses; 3+1, schedule consisting of 3 primary doses and a booster; N, total number of reported vaccine failures after the indicated schedule, including only cases with serotype information available; PCV13, 13-valent pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.
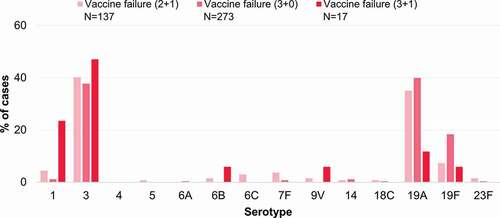
Figure 5. Vaccine failures or breakthrough IPD cases after PCV13 or PCV10 reported for each serotype in the included case-control studies. Panel a shows vaccine failure or breakthrough cases per serotype (as explained for panels b to d) as a proportion of all failure or breakthrough cases across case-control studies; N values indicate the latter. Panel b shows the number of vaccine-serotype IPD cases per study and serotype in children who received ≥1 dose of PCV13 or PCV10 (vaccine failures and breakthrough). Panel c shows the number of vaccine failures per study and serotype. Panel d shows the number of breakthrough cases per study and serotype. *Vaccine failure data were only available for children 7–24 months of age who received a 2-dose catch-up schedule. IPD, invasive pneumococcal disease; PCV13, 13-valent pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.
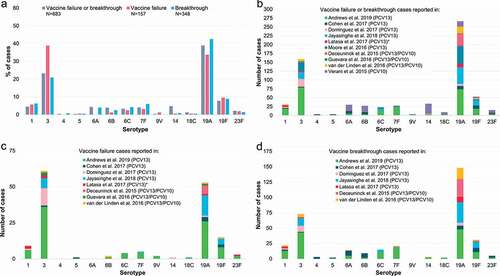
Figure 6. Percentage of the total number of vaccine failures or breakthrough IPD cases after PCV13 or PCV10 associated with each serotype. ≥1 dose refers to all vaccine-serotype IPD cases after receipt of ≥1 dose of either PCV13 or PCV10, including those that could not be classified as either vaccine failure or breakthrough (i.e. with no further information on the exact number of doses received, which was the case for two PCV13 studies: Asner et al. [Citation27], Moore et al. [Citation41], and one PCV10 study accounting for a high proportion of all cases: Verani et al. [Citation9]). IPD, invasive pneumococcal disease; N, total number of reported vaccine failures, breakthrough cases or vaccine-serotype IPD cases after receipt of ≥1 dose of either PCV13 or PCV10, including only cases with serotype information available and excluding cases where children had received a mixed PCV13/PCV10 schedule; PCV13, 13-valent pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.
![Figure 6. Percentage of the total number of vaccine failures or breakthrough IPD cases after PCV13 or PCV10 associated with each serotype. ≥1 dose refers to all vaccine-serotype IPD cases after receipt of ≥1 dose of either PCV13 or PCV10, including those that could not be classified as either vaccine failure or breakthrough (i.e. with no further information on the exact number of doses received, which was the case for two PCV13 studies: Asner et al. [Citation27], Moore et al. [Citation41], and one PCV10 study accounting for a high proportion of all cases: Verani et al. [Citation9]). IPD, invasive pneumococcal disease; N, total number of reported vaccine failures, breakthrough cases or vaccine-serotype IPD cases after receipt of ≥1 dose of either PCV13 or PCV10, including only cases with serotype information available and excluding cases where children had received a mixed PCV13/PCV10 schedule; PCV13, 13-valent pneumococcal conjugate vaccine; PCV10, pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.](/cms/asset/da4ff09f-88da-497b-8ba1-36cf2a992582/ierv_a_2012455_f0006_oc.jpg)