Figures & data
Table 1. Current meningococcal surface protein vaccines and antigens
Figure 1. Phylogenetic tree for FHbp.
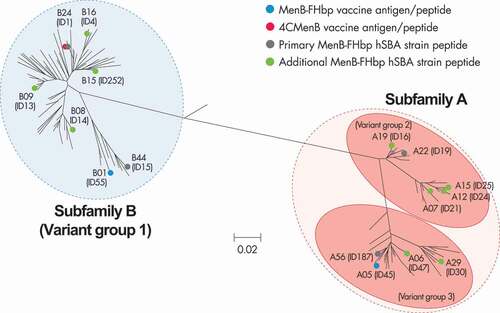
Table 2. Breadth of coverage of meningococcal surface protein vaccines: factors impacting immunogenicity and the ability of induced antibody to provide protection across diverse strains
Table 3. Meningococcal serogroup B strains frequently used in the serum bactericidal antibody assay for the analysis of 4CMenB
Table 4. Meningococcal serogroup B strains used throughout the clinical development of MenB-FHbp
Table 5. Human serum bactericidal antibody assay titers 1 month following two doses of 4CMenB on a 0–2-month schedule in adolescents 12–18 years of age against primary meningococcal serogroup B strains used throughout the MenB-FHbp clinical program [Citation97,Citation98]
Table 6. Key factors influencing laboratory assays assessing the breadth of coverage of meningococcal surface protein vaccines
