Figures & data
Table 1. Demographics and characteristics of subjects receiving BNT162b2 and mRNA-1273.
Figure 1. Comparison of anti-Spike IgG titer level at baseline (before 3rd vaccination) and Day 28 after 3rd vaccination. BNT162b2: cohort with homologous BNT162b2 prim and BNT162b2 boost vaccine. mRNA-1273: cohort with heterologous BNT162b2 prime + mRNA-1273 booster.
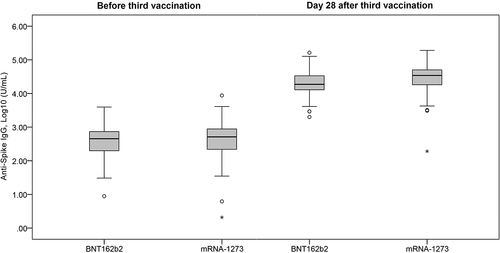
Figure 2. Distribution of anti-Spike IgG titer level at baseline (before 3rd vaccination) and Day 28 after 3rd vaccination by age groups (a) and sex (b). BNT162b2: cohort with homologous BNT162b2 prim and BNT162b2 boost vaccine. mRNA-1273: cohort with heterologous BNT162b2 prime + mRNA-1273 booster.
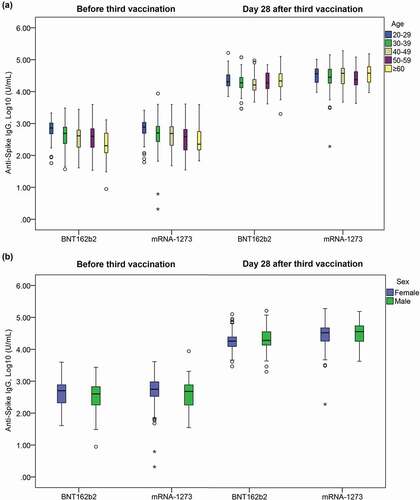
Table 2. Anti-Spike IgG at baseline and day 28 after 3rd vaccination by vaccine type, age, and sex.
Table 3. Common adverse reactions during the first 8 days after 2nd and 3rd vaccinations.
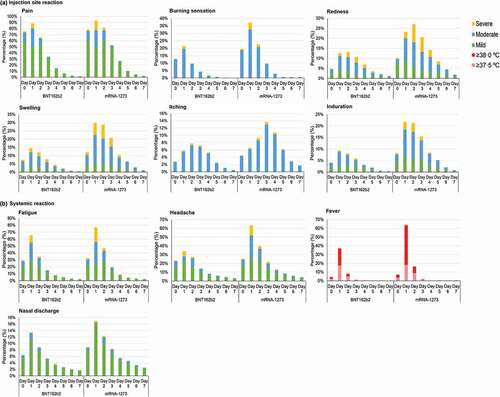
Figure 3. Distribution of self-reported adverse reactions during the first 8 days after vaccination (Day 0–7) by severity of the adverse reactions. The adverse reactions were categorized into (a) Injection-site reactions, or (b) Systemic reactions. Severity of adverse reaction were stratified into mild, moderate, or severe. Fever was stratified as ≥37.5°C and ≥38.0°C. Date of injection was defined as Day 0.
Supplemental Material
Download MS Word (353 KB)Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality, but the derived data supporting the findings of this current study are available from the corresponding author Prof. Ito and Prof. Tobita on reasonable request.