Figures & data
Table 1. Dose-ranging studies in adults aged 18–45 years (5 µg and 10 µg).
Table 2. Phase 2 and 3 early comparative studies and phase 4 investigator initiated studies.
Figure 1. Seroprotection by month (3A-HBV) in adults 18–45 years of age.
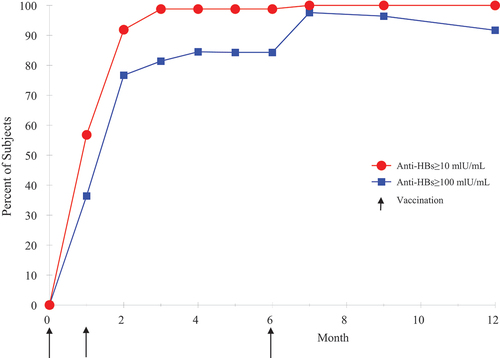
Figure 2. Seroprotection by subgroup (PROTECT).
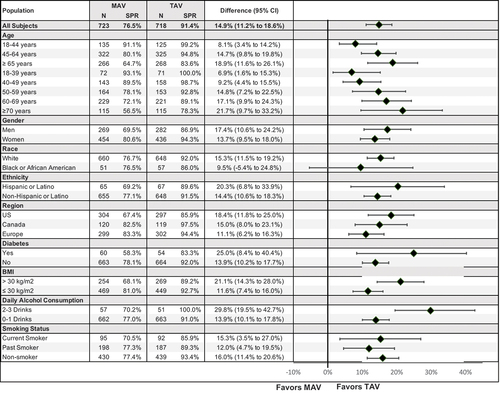
Figure 3. A. Seroprotection rate over 12 months and by age group. B. Retention of seroprotection after 2–3 years of follow-up (PROTECT).
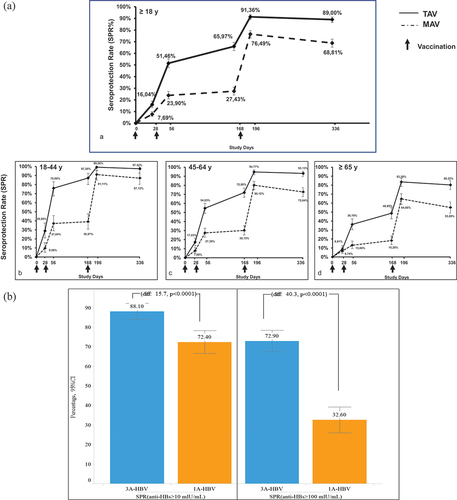
Figure 4. Geometric mean concentration anti-HBs by timepoint and type of vaccine and lot administered in the phase 3 CONSTANT study.
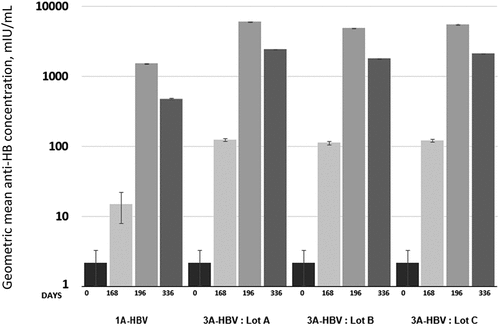
Figure 5a. Severity of local reactions by type of vaccine administered in the pivotal phase 3 studies CONSTANT and PROTECT.
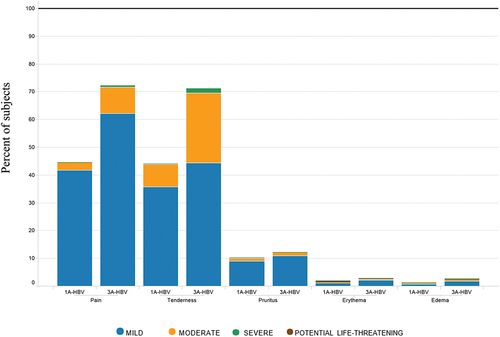
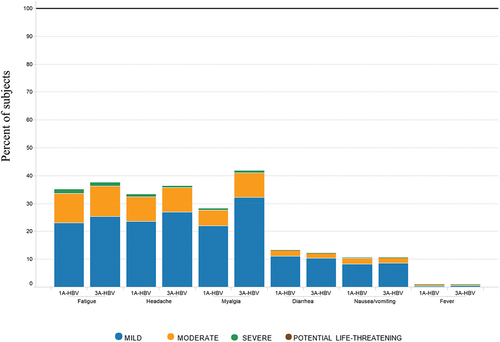
Figure 5b. Severity of systemic reactions and fever by type of vaccine administered in the pivotal phase 3 studies CONSTANT and PROTECT.