Figures & data
Figure 1. Immunological pathways for processing glycoconjugates. 1) Glycoconjugates vaccines are taken up by antigen-presenting cells (APC) and presented to B cells, which recognize glycan epitopes through the B cell receptor (BCR). 2) Upon BCR recognition, glycoconjugates are engulfed into the B cell via endocytosis. 3) In the cell, glycoconjugates are exposed to acidic conditions and further degraded to fragments by enzymes and reactive oxygen/nitrogen (ROS/RNS) species. 4) The resulting peptides and glycopeptides are loaded onto peptide-binding MHCII complexes, responsible for trafficking and exposing antigens to the cell surface. 5) As MHCII molecules can only bind peptides (and ZPS), only processed peptides were thought to be displayed at the surface for T cell recognition, however, studies have shown that T cells can also recognize carbohydrates, suggesting glycopeptides can also bind MHCII. 6) Primed T helper cells can recognize the presented antigen through their T cell receptor (TCR), and can, upon co-stimulation, initiate B cell maturation and production of memory cells and glycan-specific antibodies. In the absence of peptides, B cells cannot recruit T cell help and do not mount an efficient immune response.
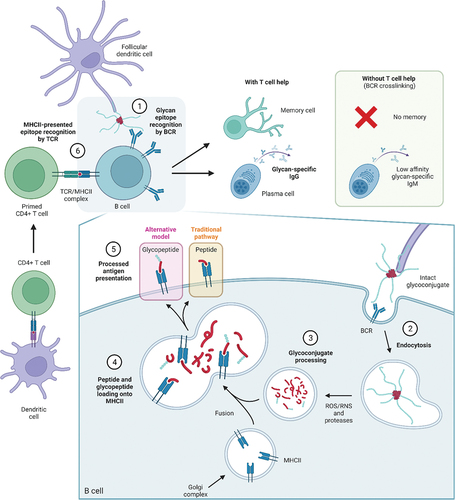
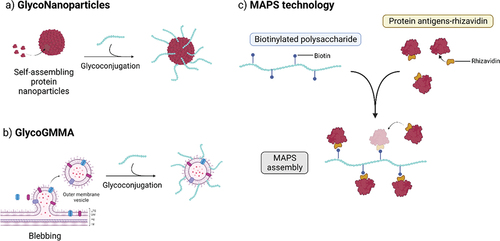
Figure 2. Simplified pathways for production of glycoconjugate vaccines. Oligosaccharides can be obtained from isolated PS through hydrolysis or oxidation. Various conjugation chemistries can be employed, resulting in site-selective or random conjugation on the carrier and/or the saccharide. Classical random conjugation strategies (a) usually involve lysine or Cysteine residues (e.g. N-hydroxysuccinimide (NHS) esters or thiol-maleimide reactions) for the protein, and hydroxyl groups for the saccharide (e.g. 1-cyano-4-dimethylaminopyridine (CDAP) activation and conjugation). Oligosaccharides obtained from PS depolymerization or chemoenzymatic methods can be conjugated at the reducing end through reductive amindation or through a linker providing a radial structure. Site-selective conjugation (b) can be achieved by glycoengineering (protein-glycan coupling techonology) or through incorporation of uAA.
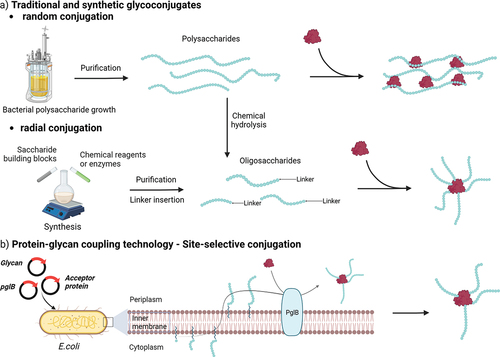
Table 1. Main advantages and drawbacks for each carbohydrate-based vaccine platform reviewed. We refer the readers to sections 3.1 and 3.2 for carbohydrate production and conjugation details.
Figure 4. a) Overview of the main surface carbohydrate classes and their surface localization on Gram-negative and -positive bacteria. b) Selected examples of polysaccharide RU and relevant oligosaccharides from ESKAPE pathogens. LPS and O-antigens structures are not shown for clarity. For WTA and LTA enterococcal structures see reference [Citation148]. Cross-reactive trisaccharide highlighted for A. baumannii CPS K3. Pel is a partially deacetylated polymer of α-1,4–GlcNAc made up predominantly of the dimeric repeat here shown [Citation149]. Alginate is composed of ManA, that can bear O-acetyls at the C2’ and/or C3’ positions, and interspersed with L-GlcA, resulting in random structures [Citation150]. Common S. aureus WTA is shown with all three possible linkages for GlcNac residues, catalyzed by glycosyltransferases TarS (β-1,4), TarM (α-1,4) and TarP (β-1,3) [Citation151]. dPNAG can be fully or partially deacetylated. All carbohydrates are represented in pyranose form, except when noted f for furanose. All carbohydrates are in D configuration (save Fuc naturally occurring in L configuration), except when stated otherwise (D/L within the symbol). At physiological pH, GlcN, GalN, ManN and Ala residues carry positive charges, while GlcA, ManA and phosphate residues carry negative charges.
![Figure 4. a) Overview of the main surface carbohydrate classes and their surface localization on Gram-negative and -positive bacteria. b) Selected examples of polysaccharide RU and relevant oligosaccharides from ESKAPE pathogens. LPS and O-antigens structures are not shown for clarity. For WTA and LTA enterococcal structures see reference [Citation148]. Cross-reactive trisaccharide highlighted for A. baumannii CPS K3. Pel is a partially deacetylated polymer of α-1,4–GlcNAc made up predominantly of the dimeric repeat here shown [Citation149]. Alginate is composed of ManA, that can bear O-acetyls at the C2’ and/or C3’ positions, and interspersed with L-GlcA, resulting in random structures [Citation150]. Common S. aureus WTA is shown with all three possible linkages for GlcNac residues, catalyzed by glycosyltransferases TarS (β-1,4), TarM (α-1,4) and TarP (β-1,3) [Citation151]. dPNAG can be fully or partially deacetylated. All carbohydrates are represented in pyranose form, except when noted f for furanose. All carbohydrates are in D configuration (save Fuc naturally occurring in L configuration), except when stated otherwise (D/L within the symbol). At physiological pH, GlcN, GalN, ManN and Ala residues carry positive charges, while GlcA, ManA and phosphate residues carry negative charges.](/cms/asset/107c52f8-5ab6-457e-a498-931321bae64a/ierv_a_2274955_f0004_oc.jpg)
Table 2. Current key preclinical carbohydrate-based approaches against ESKAPE pathogens. Use of synthetic glycan is meant by the term ‘semi-synthetic chemical conjugate’.
Table 3. Recent clinical trials for vaccines and passive immunizations against ESKAPE.
Figure 5. Incidence of AMR pathogens in adult healthcare-associated infections (US data 2015–2017). Among the ESKAPE pathogens, E. coli, S. aureus, klebsiella spp. and P. aeruginosa emerge as the ones with higher incidence across different syndromes. Vaccines against these pathogens remains a priority. Figure adapted from reference [Citation232]. Abbreviations: CAUTI, catheter-associated urinary tract infections; CLABSI, central line-associated bloodstream infections; PVAP, possible ventilator-associated pneumoniae; SSI, surgical site infections.
![Figure 5. Incidence of AMR pathogens in adult healthcare-associated infections (US data 2015–2017). Among the ESKAPE pathogens, E. coli, S. aureus, klebsiella spp. and P. aeruginosa emerge as the ones with higher incidence across different syndromes. Vaccines against these pathogens remains a priority. Figure adapted from reference [Citation232]. Abbreviations: CAUTI, catheter-associated urinary tract infections; CLABSI, central line-associated bloodstream infections; PVAP, possible ventilator-associated pneumoniae; SSI, surgical site infections.](/cms/asset/3c6f1804-c9f7-4e15-94c3-c2961299ec83/ierv_a_2274955_f0005_oc.jpg)