Figures & data
Figure 1. The observed and modeled skin microfilarial dynamics following a single dose of: (a) ivermectin (150 µg/kg) and (b) moxidectin (8 mg)
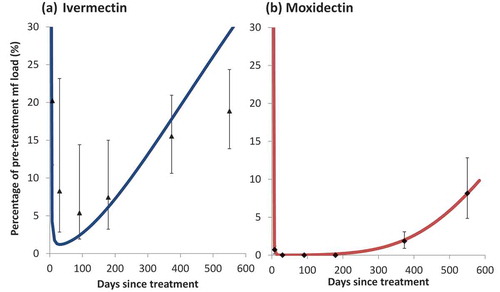
Table 1. Summary of adverse events (as a percentage of participants with AEs or SAEs) for ivermectin and moxidectin from phase II and III clinical trials
Table 2. Summary of efficacy on Onchocerca volvulus microfilariae for ivermectin and moxidectin from phase II and III clinical trials
Table 3. Characteristics of ivermectin, moxidectin, and doxycycline
