Figures & data
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of the process of study selection.
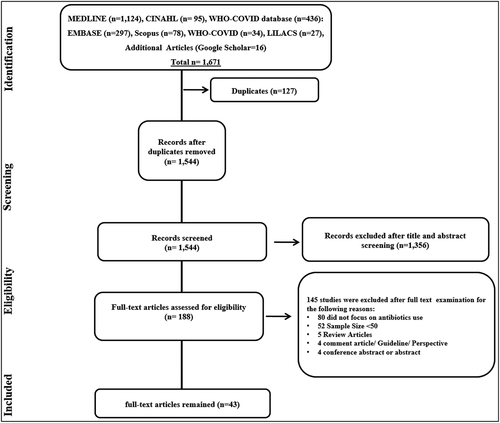
Table 1. Search strategy
Table 2. Summary of studies examining antimicrobial consumption during the COVID-19 pandemic or among COVID-19 patients
Figure 2. Quality assessment of included observational studies using Newcastle-Ottawa scale.
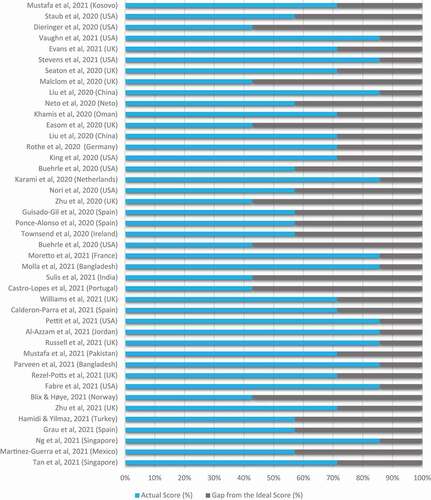
Figure 3. Pooled antimicrobial consumption estimate (%) in COVID-19 patients (Heterogeneity: I2 = 100%; p = 0.001).
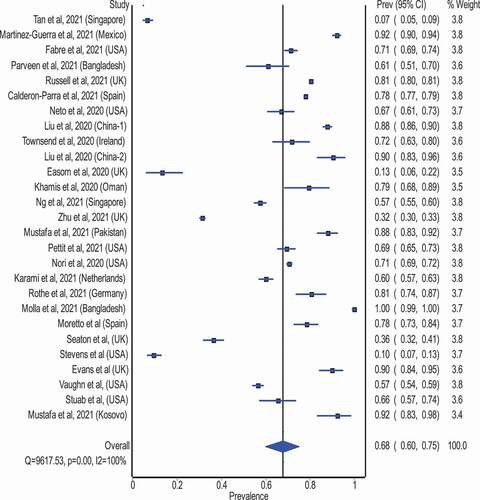
Figure 4. Pooled proportion of COVID-19 patients prescribed an antibiotic in high-income countries.
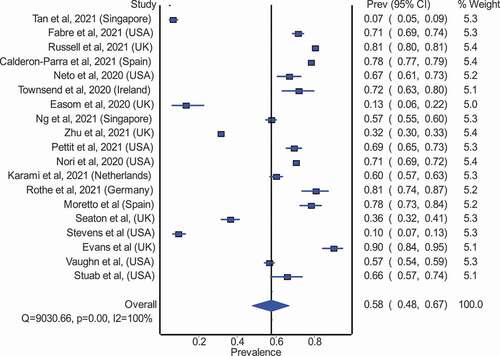
Figure 5. Pooled proportion of COVID-19 patients prescribed an antibiotic in low-middle income countries [NS: Not Specified, AMC: Antimicrobial/ antibiotic consumption, PPS: Point Prevalence Survey, PO: Per Oral, IV: Intravenous, WHO ATC: World Health Organization Anatomical Therapeutic Chemical, DDD: Defined Daily Dose, PPP: Pre-Pandemic Period, PP: Pandemic Period, ICU: Intensive Care Unit, CABP: Community-Acquired Bacterial Pneumonia, BDOC: Bed Days of Care, CAP: Community-Acquired Pneumonia, bCAP: Bacterial-Community Acquired Pneumonia, HAP/VAP: Hospital-Acquired Pneumonia/ Ventilator-Associated Pneumonia, PCT: Procalcitonin, SI: Suspected bacterial infection, NI: No evidence of bacterial infection. LRTI: Lower Respiratory Tract Infection, HCFA – CDI: Healthcare Facility-Associated Clostridioides Difficile Infection, CLABI: Central Line-Associated Bloodstream Infection, IQR: Interquartile Range, HCQ: Hydroxychloroquine, DP: Days Present].
![Figure 5. Pooled proportion of COVID-19 patients prescribed an antibiotic in low-middle income countries [NS: Not Specified, AMC: Antimicrobial/ antibiotic consumption, PPS: Point Prevalence Survey, PO: Per Oral, IV: Intravenous, WHO ATC: World Health Organization Anatomical Therapeutic Chemical, DDD: Defined Daily Dose, PPP: Pre-Pandemic Period, PP: Pandemic Period, ICU: Intensive Care Unit, CABP: Community-Acquired Bacterial Pneumonia, BDOC: Bed Days of Care, CAP: Community-Acquired Pneumonia, bCAP: Bacterial-Community Acquired Pneumonia, HAP/VAP: Hospital-Acquired Pneumonia/ Ventilator-Associated Pneumonia, PCT: Procalcitonin, SI: Suspected bacterial infection, NI: No evidence of bacterial infection. LRTI: Lower Respiratory Tract Infection, HCFA – CDI: Healthcare Facility-Associated Clostridioides Difficile Infection, CLABI: Central Line-Associated Bloodstream Infection, IQR: Interquartile Range, HCQ: Hydroxychloroquine, DP: Days Present].](/cms/asset/3ec535df-e623-438d-954a-543d89970508/ierz_a_2011719_f0005_oc.jpg)
