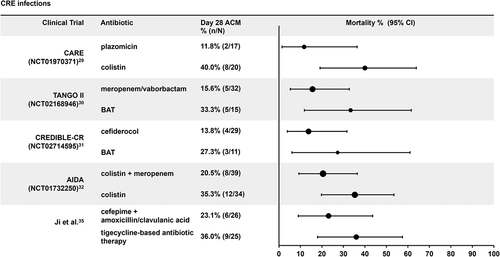
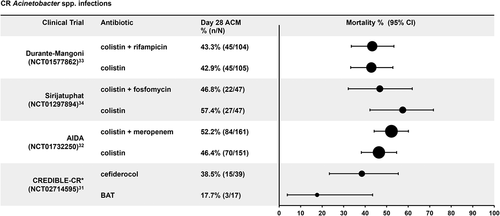
Figures & data
Table 1. Study characteristics and inclusion and exclusion criteria of studies investigating carbapenem-resistant Gram-negative infections
Table 2. Demographic and baseline details in the primary analysis populations of recent studies in adult patients with infections caused by carbapenem-resistant Gram-negative pathogens