Figures & data
Table 1. Regulatory guidance and guidelines for clinical development of antimicrobial agents.
Table 2. National and regulatory actions in Japan to promote clinical development of novel anti-infective agents.
Table 3. Key scientific proposals from the PMDA Science Board AMR subcommittee to facilitate clinical development of novel antimicrobial agents.
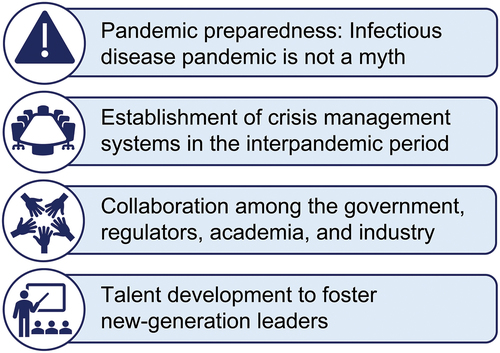
Table 4. What do we need to establish during the interpandemic period for the development of novel agents against antimicrobial-resistant bacteria?
Table 5. Push, pull, and hybrid incentive strategies for antimicrobial drug research and development.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.