Figures & data
Table 1. Potential and established presynaptic CaM targets. The table contains a list of presynaptic CaM-binding proteins from the Calmodulin Target Database (Ikura group, I [Citation15]) and from three recent CaM interactor screens (James group, J [Citation27]; Liu group, L [Citation26]; Cahill group, C [Citation28]. Proteins were selected as presynaptic based on available information in the UniProtKB database, prior knowledge, and comprehensive literature search. For each entry, we list the protein name, UniProtKB accession number, gene name, source organism (SO; Mus musculus, M; Rattus norvegicus, R; Homo sapiens, H), molecular weight (MW), and refer to the database/screen (DB/S) where the CaM interaction was described. For proteins that are directly associated with synaptic vesicles (SVs) or with the SV cycle, we also list the step in which they are presumed to exert their effect. In cases where the interaction with CaM was biochemically confirmed or where the CaM-binding site is identified, the PubMed ID (PMID) is given to indicate key papers where these findings were made.
Figure 1. Characterization of the CaM-Munc13 interaction.
(a.) Photoaffinity labeling (PAL) and chemical cross-linking (XL) complement each other in the generation of structural models. As exemplified here on the basis of the molecular modeling of the CaM/Munc13 peptide complexes, the use of photoreactive peptides containing Bpa instead of the hydrophobic anchor residue Trp or Phe (see structures for similarity of the amino acid side chains) at position 1 of the CaM-binding motif, typically reveals a low number of tight constraints, resulting in the docking solutions shown in grey (left). The contact site of the anchor residue can be positioned with high precision, whereas the peptide C-terminus can adopt many orientations due to the lack of constraints for this region. In contrast, the structural model derived from XL alone (right) has less degrees of freedom with regard to ligand orientation and overall structure, indicating that the increase in constraint number balances the increase in constraint length. Nevertheless, the absence of tight constraints from PAL hampered the exact localization of the peptide N-terminus as indicated by a relatively high deviation from the best-matching structure in this region (CaM, green; nNOS peptide, red; PDB 2O60). High-quality structural models were finally obtained when the two complementary sets of constraints from PAL and XL were combined and led to the conclusion that all Munc13 isoforms employ a common mode of binding, despite a lack of sequence homology between their CaM-binding sites. In the sequence alignment, bold font represents the peptides used in the initial cross-linking experiments. Residues homologous to Munc13-1 are underlined. Position 1 of the CaM-binding motifs is marked by an asterisk. Structural models were modified from [Citation51,Citation90].(b.) Sequence alignment of C-terminally extended CaM-binding motifs in unc-13 proteins from different species. Hydrophobic anchor residues in motif position 1, 5, 8 (red) and 26 (green) are marked if present. Residues homologous to Munc13-1 from Homo sapiens are underlined. The following protein sequences were used (NCBI accession in parentheses): Homo sapiens, protein unc-13 homolog A (NP_001073890); Rattus norvegicus, protein unc-13 homolog A (NP_074052); Mus musculus, protein unc-13 homolog A (NP_001025044); Danio rerio, protein unc-13 homolog A (NP_001038630); Caenorhabditis elegans, UNC-13 (AAA93094); Drosophila melanogaster, unc-13 isoform A (NP_651949).
![Figure 1. Characterization of the CaM-Munc13 interaction.(a.) Photoaffinity labeling (PAL) and chemical cross-linking (XL) complement each other in the generation of structural models. As exemplified here on the basis of the molecular modeling of the CaM/Munc13 peptide complexes, the use of photoreactive peptides containing Bpa instead of the hydrophobic anchor residue Trp or Phe (see structures for similarity of the amino acid side chains) at position 1 of the CaM-binding motif, typically reveals a low number of tight constraints, resulting in the docking solutions shown in grey (left). The contact site of the anchor residue can be positioned with high precision, whereas the peptide C-terminus can adopt many orientations due to the lack of constraints for this region. In contrast, the structural model derived from XL alone (right) has less degrees of freedom with regard to ligand orientation and overall structure, indicating that the increase in constraint number balances the increase in constraint length. Nevertheless, the absence of tight constraints from PAL hampered the exact localization of the peptide N-terminus as indicated by a relatively high deviation from the best-matching structure in this region (CaM, green; nNOS peptide, red; PDB 2O60). High-quality structural models were finally obtained when the two complementary sets of constraints from PAL and XL were combined and led to the conclusion that all Munc13 isoforms employ a common mode of binding, despite a lack of sequence homology between their CaM-binding sites. In the sequence alignment, bold font represents the peptides used in the initial cross-linking experiments. Residues homologous to Munc13-1 are underlined. Position 1 of the CaM-binding motifs is marked by an asterisk. Structural models were modified from [Citation51,Citation90].(b.) Sequence alignment of C-terminally extended CaM-binding motifs in unc-13 proteins from different species. Hydrophobic anchor residues in motif position 1, 5, 8 (red) and 26 (green) are marked if present. Residues homologous to Munc13-1 from Homo sapiens are underlined. The following protein sequences were used (NCBI accession in parentheses): Homo sapiens, protein unc-13 homolog A (NP_001073890); Rattus norvegicus, protein unc-13 homolog A (NP_074052); Mus musculus, protein unc-13 homolog A (NP_001025044); Danio rerio, protein unc-13 homolog A (NP_001038630); Caenorhabditis elegans, UNC-13 (AAA93094); Drosophila melanogaster, unc-13 isoform A (NP_651949).](/cms/asset/0ef9ffe7-f70d-447a-b789-f801d7c02433/ieru_a_1275966_f0001_oc.jpg)
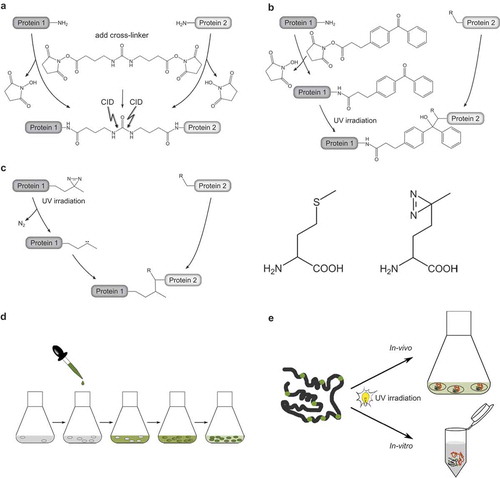
Figure 2. Investigation of CaM/peptide complexes using ion mobility-mass spectrometry.
(a.) Collision cross sections (CCSs) of CaM and CaM/peptide complexes as a function of their protein mass. CCSs of the compact and dumbbell conformation of CaM are shown as open squares. The estimated mass-to-CCS correlation of idealized spherical particles and idealized cylindrical particles are shown as black and grey lines, respectively. The trendlines provide an indication if the investigated complexes formed by CaM and different peptides (P1, P2, P3) are of the compact or extended type.(b.) Amino acid sequences of the investigated peptides skMLCK577-602 (M13) and skMLCK575-607 (skMLCK). Hydrophobic anchor residues of the 1–5–8–14 CaM-binding motif position are marked in red, while a potential hydrophobic contact site in motif position 26 of skMLCK is marked in green.(c, d.) Negative ion mass spectra of CaM/peptide complexes. Spectra were derived from 10 µM CaM without external addition of Ca2+ and in presence of 20 µM M13 (c) and 20 µM skMLCK (d), respectively. Charge states of unliganded CaM are shown in black, whereas the charge states of the CaM/M13 and the CaM/skMLCK complex are highlighted in red and blue, respectively. In all cases the ratio of bound complex is Ca2+/CaM/peptide 4:1:1.(e.) Arrival time distributions (ATDs) and experimental CCSs. Shown are helium CCSs (DTCCSHe) of CaM and the corresponding CaM/peptide complexes with four Ca2+ in charge state 8- and 9-. Note that in both charge states, the experimental DTCCSHe of the CaM/skMLCK complex does not differ significantly from that of the CaM/M13 complex, identifying both CaM/peptide complexes as compact type.
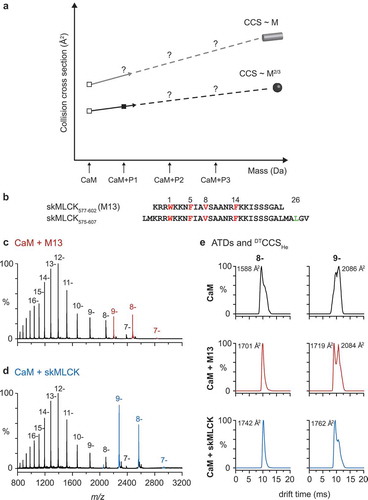
Figure 3. Reagents and strategies for protein-protein cross-linking.
(a.) Homobifunctional, MS-cleavable BuUrBu-linker containing two amine-reactive N-hydroxysuccinimid (NHS) esters. The linker reacts with primary amine groups in Lys residues creating an amide bond. Under CID conditions the central urea moiety is cleaved generating a characteristic fragment ion signature.(b.) Heterobifunctional, amine/photo-reactive cross-linker N-succinimidyl-p-benzoyldihydrocinnamate (SBC). Cross-linking reactions with SBC are typically conducted in a two-step fashion. First, the amine-reactive site of SBC (NHS ester) is allowed to react with protein 1 and non-reacted reagent can be quenched and removed. Second, SBC-labeled protein one is incubated with protein 2 and activation of the photo-reactive site of SBC (benzophenone moiety) by UV irradiation leads to the formation of covalent bonds via a diradicalic mechanism.(c.) Photo-Met contains a diazirine that is activated by UV irradiation. Consequently, a carbene is created that forms a covalent bond with N-H and C-H groups of an amino acid in spatial proximity. Note the structural similarity of Met (left) and photo-Met (right), the latter containing a diazirine group instead of the sulfur atom.(d.) Incorporation strategy of photo-Met into proteins in E. coli cells. Cells are grown in mineral salts medium before photo-Met is directly added to the medium. Photo-Met is incorporated into proteins during protein synthesis.(e.) In vivo and in vitro approach for protein interaction studies using photo-Met.