Figures & data
Table 1. Proteomic Studies of Senescent Cells.
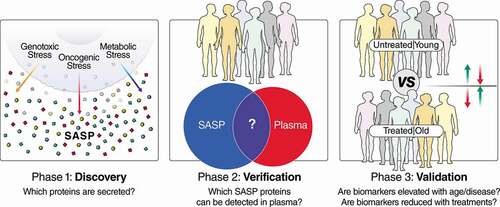
Figure 1. Pipeline for the development of senescence-based biomarkers of aging and disease. In phase 1, discovery, the secretomes (SASP) of senescent cells are characterized in cell-culture systems to generate biomarker candidates that can be tailored to specific biological contexts, based on the disease and cell type. Multiple cell types and senescence-inducing stimuli (e.g., genotoxic/oncogenic/metabolic stress) can be interrogated, and the most robust biomarkers of all conditions used for later stages of biomarker development. In step 2, verification, biomarker candidates are filtered, based on whether they can be detected in plasma (or other biofluids), and biomarker assays are developed based on the most reproducible and quantitative peptide(s) from each protein biomarker candidate. Finally, these biomarkers are interrogated in human cohorts in step 3, validation. Cohorts for step 3 should be chosen so that they contain subjects with high and low senescent cell burdens, and ideally, an intervention that either increases or reduces senescent cell burden. Examples of cohorts with variable senescence burden are old and young individuals, and doxorubicin-treated patients and untreated patients (or before and after doxorubicin treatment).