Figures & data
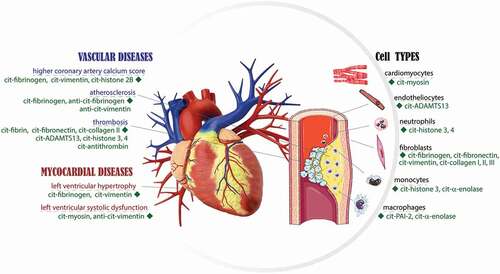
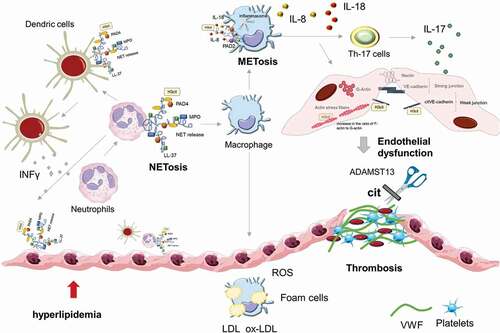
Table 1. Reported PADs isoforms, citrullinated proteins and mechanisms, in cardiovascular experimental data sets