Figures & data
Table 1. Characteristics of patient hospitalized for febrile neutropenia (patients baseline characteristics are reported per patients, while other characteristic such as ECOG or treatment intent, are reported per episode of febrile neutropenia, as they may evolve for patient presenting with multiple episodes of febrile neutropenia).
Table 2. Most common regimens associated with febrile neutropenia at our institution.
Figure 1. Comparison of febrile neutropenia rates in the cohort with data from clinical trials.
AUC: Area under the curve; BEP: Bleomycin 30 mg D1 D8 D15, etoposide 100 mg/m2 D1-D5, cisplatin 20 mg/m2 D1-D5, pegfilgrastim 6 mg D6, q3W; Carboplatin etoposide: Carboplatin AUC 5 D1, etoposide 100 mg/m2 D1-D3 q3w; Ciplatin etoposide: Cisplatin 100 mg/m2 D1, etoposide 100 mg/m2 D1-D3 q3w; EC: Cyclophosphamide 600 mg/m2 D1, Epirubicin 90 mg/m2 D1 q3w; FLOT: 5-FU 2000 mg/m2 over 24 h, leucovorin 30 mg D1, Docetaxel 50 mg/m2 D1, oxaliplatin 85 mg/m2 D1 q2w; R-CHOP: Rituximab 375 mg/m2 D1, cyclophosphamide 750 mg/m2 D1, doxorubicin 50 mg/m2 D1, vincristine 1.4 mg/m2 D1, prednisone 40 mg/m2 D1-D5; R-DA-EPOCH: Rituximab 375 mg/m2 D1, dose-adjusted etoposide 50–124 mg/m2 (depending on dose level) D1-D5, prednisone 120 mg/m2 D1-D5, vincristin 0.4 mg/m2 D1-D4, cyclophosphamide 750–1866 mg/m2 (depending on dose level) D5, doxorubicin 10–25 mg/m2 (depending on dose level) D1-D4, filgrastim 0.5 mioU/kg starting from D6 until absolute neutrophile count >5000 cells/ul, q3w; RTX-cisplatin: Cisplatin 100 mg/m2 D1 q3w with concurrent radiotherapy.
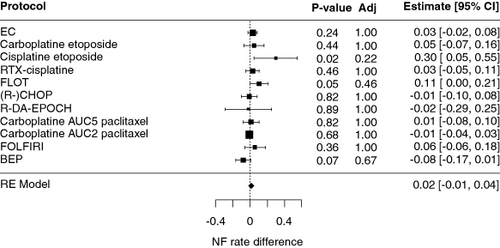
Table 3. Among patients hospitalized for febrile neutropenia, potential drug interaction between chemotherapy and comedications: associations that are usually contra-indicated.
Table 4. Patients hospitalized for febrile neutropenia who presented significant abnormal pre-treatment laboratory values, and the regimen they received, with dose-adjustment.
Supplementary Figure S1
Download PNG Image (219.6 KB)Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
