Figures & data
Table 1. Eligibility criteria that were specific to QUIET-1, including a history of approved substantial changes to the Clinical Trial Protocol.
Table 2. Eligibility criteria that were specific to CLARITY-1, including a history of approved substantial changes to the Clinical Trial Protocol.
Figure 1. Recruiting sites for QUIET-1 in England (left-hand panel) and for CLARITY-1 in the US (right-hand panel).
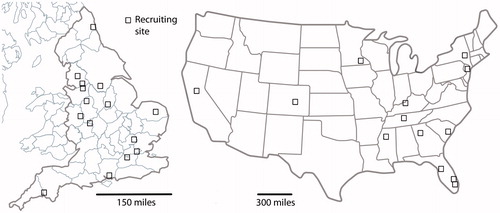
Table 3. Timeline for opening sites to QUIET-1 recruitment, with screening and randomisation listings.
Table 4. Timeline for opening sites to CLARITY-1 recruitment, with screening and randomisation listings.
Table 5. Efficiency of the different recruitment methods used in the QUIET-1 and CLARITY-1 RCTs.
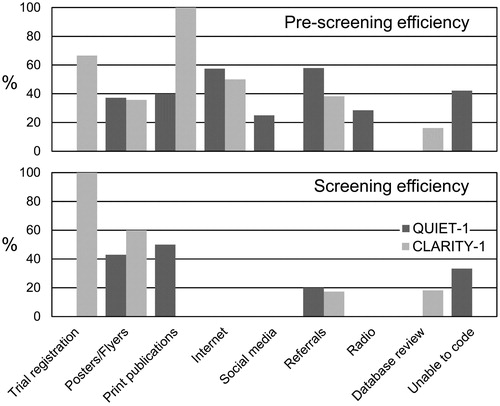
Figure 2. Efficiency of the different recruitment methods used in the QUIET-1 and CLARITY-1 RCTs. The top panel shows pre-screening efficiency measured by determining the number of people eligible for screening as a percentage of those who underwent telephone pre-screening. The bottom panel shows screening efficiency measured by the number of randomised participants as a percentage of those screened.