Figures & data
Figure 1. Sequence alignment of human TMPK isoforms. Accession numbers: Isoform 1 (Np_036277.2), isoform 2 (Np_001158503.1), isoform 3 (Np_001307831.1), isoform 4 (Np_001307832.1), isoform 5 (Np_001307833.1), isoform 6 (Np_001307834.1). Important functional motifs are marked. The structure-based sequence alignment was performed by using the ESPript 3.0 program.[Citation15]
![Figure 1. Sequence alignment of human TMPK isoforms. Accession numbers: Isoform 1 (Np_036277.2), isoform 2 (Np_001158503.1), isoform 3 (Np_001307831.1), isoform 4 (Np_001307832.1), isoform 5 (Np_001307833.1), isoform 6 (Np_001307834.1). Important functional motifs are marked. The structure-based sequence alignment was performed by using the ESPript 3.0 program.[Citation15]](/cms/asset/df68998a-36d3-4303-aa33-a7a967ad824f/lncn_a_2023748_f0001_c.jpg)
Figure 2. Human TMPK structure (isoform 1). (A) The human TMPK structure shown as surface model. The helices are labeled with cyan, the β-sheet with red and loops are labeled with magenta. In the ligand-binding pocket: ATP is located partly on the surface and dTMP binds in the interior of the protein. (B) Human TMPK structure shown as a cartoon. Residues interacting with dTMP: hydrophobic interaction with F72, F105 and Y151, labeled in blue, the side chain of R76 binds to the thymine ring and the side chain of R97 interacts directly with the phosphate group of dTMP via a H-bond. (C) The β-sheet structure of human TMPK. The five β-strands are placed in the same direction and form the backbone of the protein structure. The human TMPK structure used in the analysis was from https://www.rcsb.org/ (pdb code: 1E2F).
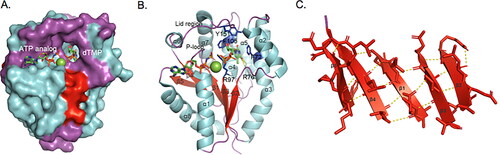
Figure 3. Structure model of TMPK isoform 6. (A) Human TMPK (isoform 1) homodimer. The α3 makes interface between the two monomers, through hydrophobic interactions (in green); (B) Homology model of the TMPK isoform 6 monomer. The 39-amino acids insertion is shown as a loop (salmon). (C) Structural model of the TMPK isoform 6 dimer. (D) Superimposed structures of isoform 1 and 6. Isoform 1 is in cyans and isoform 6 is in firebrick color. The 39 amino acid insertion is in green. Structural modeling was performed at https://swissmodel.expasy.org/ with human TMPK structure as template (PDB code: 1E2F).
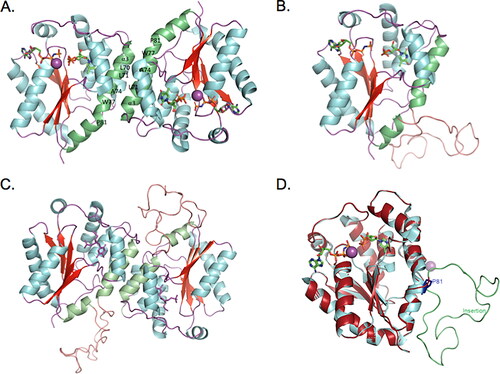
Table 1. Summary of structural analyses of human TMPK isoforms.
Figure 4. Functional characterization of TMPK isoform 6. (A) Purified TMPK isoform 6 and isoform 1. Lane 1, E. coli extracts of uninduced culture; lane 2: E. coli extracts of induced culture for isoform 6; lane 3 to 7, correspond to fractions 1-5 of the purified isoform 6; lane 8, purified isoform 1. (B) Size-exclusion chromatography of purified isoform 6. Fractions were collected and assayed for TMPK activity using [3H]-dTMP as substrate and shown as pmol/min/ml. Selected fractions were analyzed by SDS-PAGE and western blot using a human TMPK specific antibody. The elution positions of molecular weight standards are indicated. (C) TMPK activity. Recombinant isoform 1 and 6 were assayed for enzymatic activity using [3H]-dTMP as substrate; data are from three independent measurements and given as mean ± SD; (D) Western blot analysis using a human TMPK-specific antibody. Lane 1, recombinant human TMPK (isoform 1), Lane 2, extracts from fibroblasts.
![Figure 4. Functional characterization of TMPK isoform 6. (A) Purified TMPK isoform 6 and isoform 1. Lane 1, E. coli extracts of uninduced culture; lane 2: E. coli extracts of induced culture for isoform 6; lane 3 to 7, correspond to fractions 1-5 of the purified isoform 6; lane 8, purified isoform 1. (B) Size-exclusion chromatography of purified isoform 6. Fractions were collected and assayed for TMPK activity using [3H]-dTMP as substrate and shown as pmol/min/ml. Selected fractions were analyzed by SDS-PAGE and western blot using a human TMPK specific antibody. The elution positions of molecular weight standards are indicated. (C) TMPK activity. Recombinant isoform 1 and 6 were assayed for enzymatic activity using [3H]-dTMP as substrate; data are from three independent measurements and given as mean ± SD; (D) Western blot analysis using a human TMPK-specific antibody. Lane 1, recombinant human TMPK (isoform 1), Lane 2, extracts from fibroblasts.](/cms/asset/d0994f41-abb8-43ca-8b8e-24f10fb67ebe/lncn_a_2023748_f0004_c.jpg)
