Figures & data
Figure 1. SAMHD1(113-626), a truncation lacking the N-terminal SAM domain, retains dNTP hydrolysis activity. (a) Domain schematic of full-length (FL) human SAMHD1 and the truncation removing the N-terminal SAM domain (SAMHD1 113-626). (b) SDS-PAGE analysis of SAMHD1 FL and SAMHD1(113-626). Representative (n = 2) image shown. (c) SAMHD1 FL and SAMHD1(113-626) have comparable dGTP hydrolysis activity in the enzyme-coupled activity assay. Schematic of assay is shown. SAMHD1 FL and SAMHD1(113-626) were incubated with a range of dGTP concentrations for 20 min before the reaction was stopped and dGTP hydrolysis measured. Average values from 3 independent experiments (each performed in quadruplet) shown. Sigmoidal substrate-velocity curves were fitted using the allosteric enzyme kinetic model in Prism 10 (GraphPad Software).
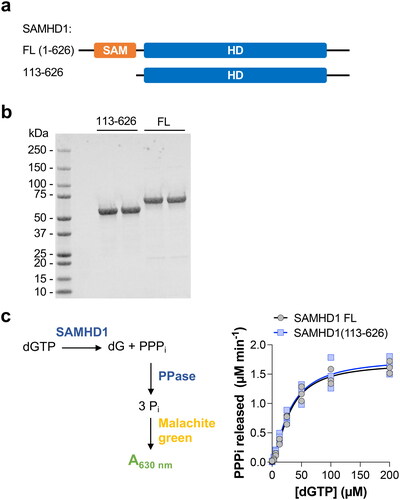
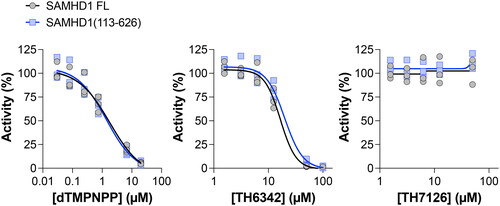
Figure 2. The N-terminal SAM domain of the dNTPase SAMHD1 is not required for inhibition by small molecule TH6342. SAMHD1 full-length (FL) or SAMHD1(113-626), the truncation lacking the N-terminal SAM domain, were incubated with a titration of non-hydrolysable dNTP analogue dTMPNPP, SAMHD1 inhibitor TH6342, or analogue control TH7126, in the enzyme coupled activity assay. Average inhibition values, normalised to solvent controls, from 3 independent experiments each performed in quadruplet shown. Dose-response curves (four-parameter, variable slope) were fitted using Prism 10 (GraphPad Software).