Figures & data
Table 1 Model outcomes of interest.
Table 2 Model inputs
Table 3 Pre-trial outcomes: Pre-trial results from probabilistic sensitivity analysis (PSA) of the treatment comparisons included in alternative trials
Table 4 Value of Information of alternative trials: comparing “three-pill generic versus single-pill branded regimens” to “two-pill generic versus single-pill branded regimens”
Figure 1 Distribution of post-trial optimal strategies of alternative trials. (A) depicts the distribution of post-trial optimal strategies of the head-to-head trial of three-pill generic ART versus single-pill branded ART. For a given willingness to pay (WTP) threshold (horizontal axis), the proportion of PSA runs (vertical axis) of which three-pill generic ART strategy and single-pill branded ART strategy has the highest net monetary benefit (NMB) after ascertaining perfect information on ART efficacy for both treatment arms in the trial are represented by the red and green curves, respectively. Net monetary benefit was defined as QALY times WTP, minus cost. The dotted vertical line represents the WTP threshold at baseline ($100 000/QALY). (B) depicts the distribution of post-trial optimal strategies of the head-to-head trial of two-pill generic ART versus single-pill branded ART. For a given WTP threshold (horizontal axis), the proportion of PSA runs (vertical axis) of which two-pill generic ART strategy and single-pill branded ART strategy has the highest NMB after ascertaining perfect information on ART efficacy for both treatment arms in the trial are represented by the blue and green curves, respectively. NMB was defined as QALY times WTP, minus cost. The dotted vertical line represents the WTP threshold at baseline ($100 000/QALY).
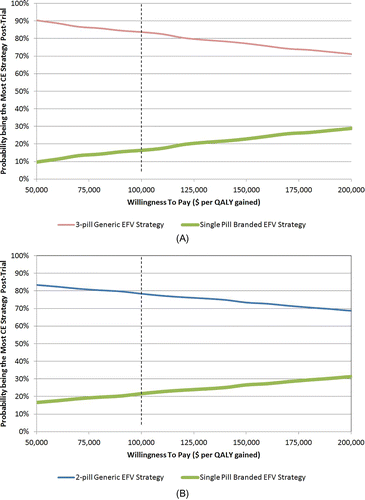
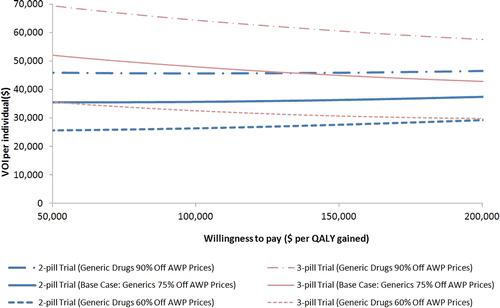
Figure 2 Direct comparison of the value of information (VOI) of alternative trials in relation to generic drug price discount and willingness to pay (WTP) per QALY. The value of ascertaining perfect information per person of ART efficacy from the three-pill trial (for both three-pill generic and single-pill branded treatment arms) and the two-pill trial (for both two-pill generic and single-pill branded treatment arms) are represented by the red and blue solid curves, respectively. The VOI per person was estimated as the difference in net monetary benefit (NMB) between single-pill branded (the pre-trial initial strategy of choice) and the post-trial optimal first line ART strategies. Net monetary benefit was defined as QALYs times WTP, minus cost. Further, the graph shows the changes in the VOI per person of a three-pill trial (red curves) and a two-pill trial (blue curves) while the generic drug discount from the AWP is varied from 60% (evenly dotted) to 90% (unevenly dotted), in comparison to the base case where generic drug discount was 75% (solid).