Figures & data
Figure 1 Study design.
ARV, antiretroviral; ATV, atazanavir; DRV, darunavir; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; r, low-dose ritonavir; qd, once daily. a29 subjects due to over-enrollment. b14 subjects due to over-enrollment. c15 subjects due to over-enrollment. Two subjects withdrew before treatment initiation.
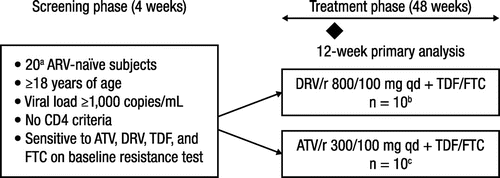
Table 1 Subject disposition
Table 2 Demographics and baseline characteristics of subjects who initiated therapy
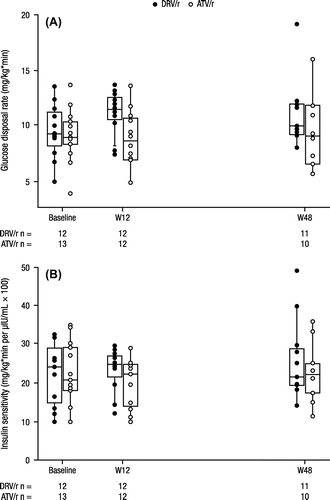
Figure 2 Clamp analyses at baseline, week 12, and week 48: (A) glucose disposal rate and (B) insulin sensitivity.a
DRV, darunavir; r, low-dose ritonavir; ATV, atazanavir; W12, week 12; W48, week 48; IQR, interquartile range. aThe boxes and horizontal lines reflect the IQR and median values, respectively. The vertical lines reflect the 5th and 95th percentiles. There is one outlier in the DRV/r group at the week 48 time point. The week 12 insulin sensitivity value of 174.8 mg/kg*min per μIU/mL × 100 in the ATV/r group is not displayed (outlier).