Figures & data
Figure 1 STRATEGY-NNRTI trial profile and disposition of subjects at Week 96
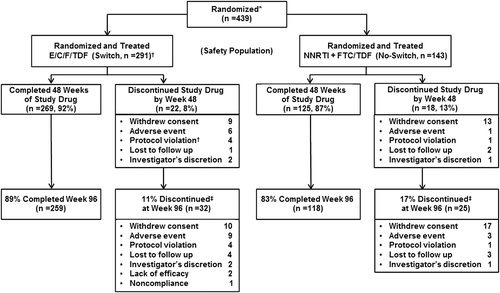
Table 1 Analysis of efficacy (HIV-1 RNA < 50 copies/mL) at Week 48 and Week 96 (Snapshot)
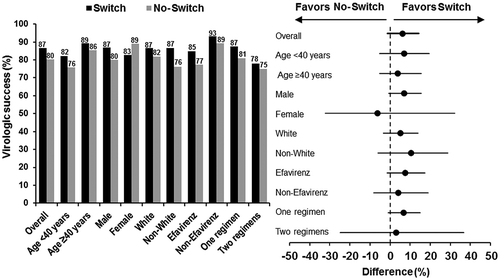
Figure 2 Analysis of efficacy (HIV-1 RNA < 50 copies/mL, Snapshot) by baseline subgroup