Figures & data
FIGURE 1. Characterization of MAb EM-31 binding to glycated prion proteins by Western blot and ELISA. (a) Three identical blots developed with EM-31, control prion antibody 6H4, or stained for proteins with colloidal silver (Ag). Lines: 1, normal mouse brain homogenate (mBh); 2, normal human brain homogenate (hBh); 3, recombinant mouse PrP (rmPrP); 4, glycated rmPrP (rmPrP-CML); 5, recombinant human PrP (rhPrP); 6, glycated rhPrP (rhPrP-CML); 7, glycated BSA (BSA-CML). EM-31 reacts only with glycated rPrPs. (b) Two identical blots developed with MAb EM-31 and control prion MAb AG4. Lines: 8, reduced rmPrP-CML; 9, reduced rmPrP-AGE (glycated by incubation with ribose); 10, nonreduced rmPrP after incubation with ribose in Tris buffer (glycation inhibited); 11, glycated nonreduced rmPrP after incubation with ribose in phosphate buffer. Reactivity of EM-31 with rmPrP-CML and rmPrP-AGE is lost after reduction of samples with dithiothreitol. (c) EM-31 reacts with C-terminal fragments of rhPrP-CML molecule: 23–231 (lane 12), 81–231 (lane 13), 90–231 (lane 14), and 121–231 (lane 15). (d) Time course of rmPrP modification by incubation with 1 M D-ribose detected by EM-31. (e) EM-31 has only a weak affinity to PrP in glycated human brain homogenate (hBh-CML) (lane 16). MAb 3F4 is used as a control of glycation. It binds to PrP in nonglycated hBh (lane 17), but not in hBh-CML (lane 18). MAb AG4 is a glycation insensitive control detecting PrP in hBh-CML (lane 19). (f) Demonstration of the specificity of EM-31 for glycated prion protein by ELISA. Wells of the plate were coated by rhPrP, rmPrP, rhPrP-CML, rmPrP-CML, rmPrP-AGE, or BSA-CML and developed with increasing concentrations of MAbs: EM-31 (rectangles, dotted line), glycation-sensitive 3F4 (triangles, dashed line), and glycation-insensitive AG4 (circles, full line). Labeling in rmPrP-AGE figure is AG4 (circles, full line), 3F4 (inverted triangles, dashed line), and two batches of EM-31 (rectangles, dashed and dotted line, and triangles, dashed and double dotted line). EM-31 does not bind to unmodified proteins and the reactivity of 3F4 to rhPrP is lost after its modification. Neither MAb binds to glycated BSA.
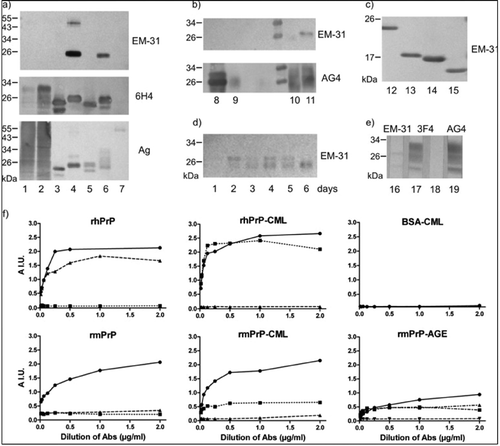
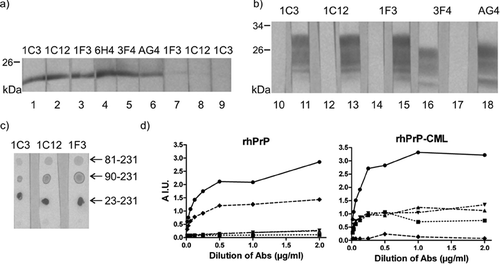
FIGURE 2. Three hybridoma supernatants demonstrate affinity to PrP in the in vitro glycated human brain homogenate. (a) Western blot of rhPrP was cut in strips and strips 1–3 were glycated by incubation with glyoxylic acid while strips 4–9 were left untreated. Supernatants 1C3, 1C12, and 1F3 demonstrate strong reactivity with the PrP on treated strips (1–3), but only faint reactivity with untreated rhPrP (7–9). The presence of the rhPrP is confirmed by MAbs 6H4, 3F4, and AG4 (4–6). (b) Clones 1C3, 1C12, and 1F3 recognize PrP in the in vitro glycated human brain homogenate (hBh-CML, lanes 11, 13, 15), but not in untreated hBh (lanes 10, 12, 14). MAb 3F4 is used as a control for glycation. It binds to PrP in nonglycated hBh (lane 16), but not in hBh-CML (lane 17). MAb AG4 is a positive control for PrP in hBh-CML (lane 18). (c) The reactivity of all three clones with glycated C-terminal fragments rhPrP81–231 and rhPrP90–231 on dot-blots is weaker than with rhPrP23–231-CML. (d) Demonstration of 1C3, 1C12, and 1F3 reactivity with rhPrP-CML by ELISA. Wells of the plate were coated with rhPrP or rhPrP-CML and developed with increasing dilutions of MAb: 6H4, glycation insensitive (circle, full line); 3F4, glycation sensitive (diamond, dashed line); 1C3 (inverted triangle, dashed and double dotted line); 1C12 (triangle, dashed and dotted line); and 1F3 (rectangle, dotted line). Reactivity of 3F4 after glycation is lost, while the reactivity of the MAbs produced by the tested clones increases.