Figures & data
Figure 1. Histogram of indentation modulus approximated by the superposition of three lognormal distributions; after [Citation3].
![Figure 1. Histogram of indentation modulus approximated by the superposition of three lognormal distributions; after [Citation3].](/cms/asset/be5d362b-ed7d-48e4-a04a-0b8abd15f1c3/umcm_a_2073493_f0001_c.jpg)
Table 1. Results obtained from grid nanoindentation testing [Citation3]: values of medians, modes, and volume fractions associated with the three lognormal distributions representing the histogram of indentation moduli in .
Table 2. Input quantities of the solid material constituents: bulk moduli, ki, shear moduli gi, as well as lognormal parameters μi and σi which are consistent with median and mode values listed in ; input values taken from [Citation3, Citation17, Citation18].
Figure 2. Micromechanical representation of Biodentine (“material organogram”): the two-dimensional sketch shows qualitative properties of a three-dimensional representative volume element of the lognormal microelasticity model which accounts for stiffness distributions of two populations of hydrates.
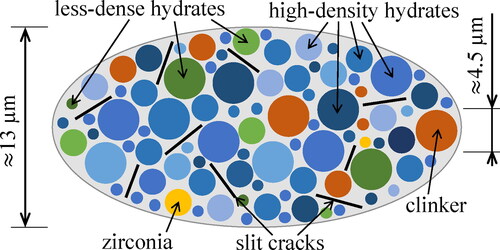
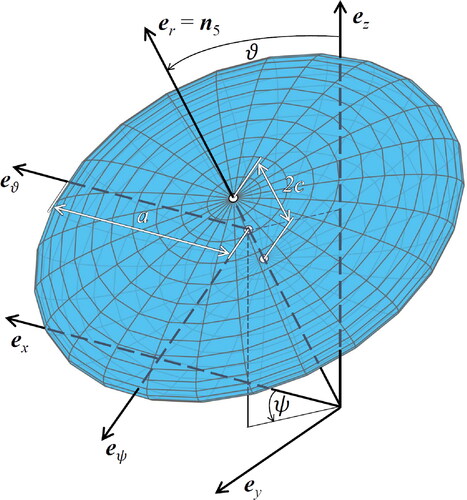
Figure 3. Eshelby-type matrix inclusion problems: (a) spherical inclusion, and (b) oblate spheroid, both embedded in an infinite matrix with isotropic stiffness , subjected remotely to auxiliary uniform strains
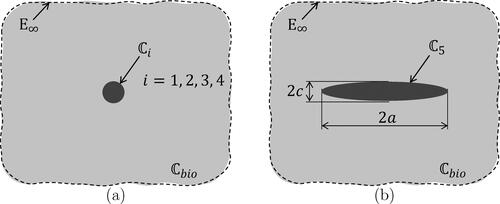
Figure 5. (a) Bulk and (b) shear modulus of the hydrates as a function of the indentation modulus, for three different values of Poisson’s ratio of the hydrates.
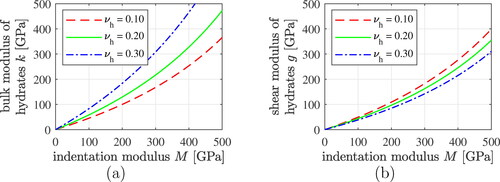
Table 3. Flowchart for the computation of kbio and gbio according to EquationEqs. (41)(41)
(41) and Equation(42)
(42)
(42) , respectively.
Figure 6. Square root of the sum of squared errors (59), quantifying the difference between computed homogenized stiffness moduli, see EquationEqs. (41)(41)
(41) and Equation(42)
(42)
(42) as well as , and their experimental counterparts, see EquationEqs. (1)
(1)
(1) and Equation(2)
(2)
(2) .
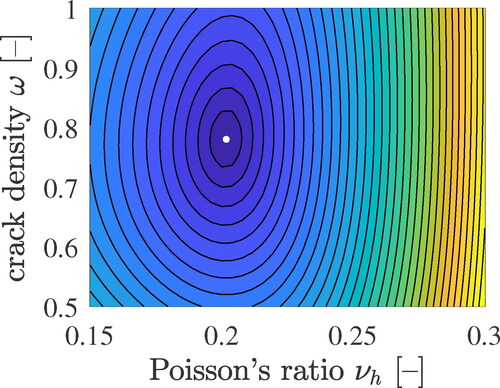
Figure 7. Homogenized stiffness moduli kbio and gbio according to EquationEqs. (41)(41)
(41) and Equation(42)
(42)
(42) , as functions of the crack density of Biodentine and Poisson’s ratio of the hydrates, see the solid blue lines, and ultrasonics-derived counterparts according to EquationEqs. (1)
(1)
(1) and Equation(2)
(2)
(2) , see the red dashed lines; the circles mark the optimal solutions, see also EquationEqs. (60)
(60)
(60) and Equation(61)
(61)
(61) .
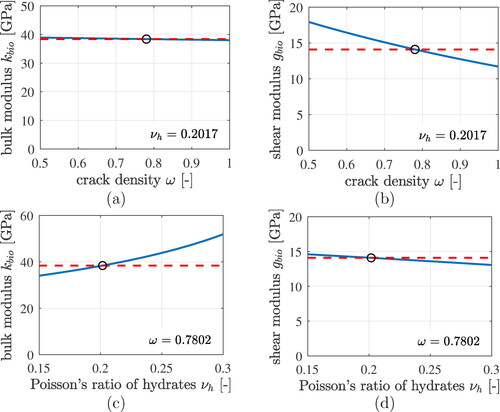
Figure 8. Results of the lognormal microelasticity model (black graphs): statistical distributions of volumetric and deviatoric components of the strain concentration tensors of LDCR hydrates and of HDCR hydrates: (a) and (c) show probability density distributions, (b) and (d) cumulative distribution functions. The best fits of generalized beta-distributions to the statistical distributions are the red dashed graphs, see EquationEqs. (70)(70)
(70) and Equation(71)
(71)
(71) as well as the Beta distributions parameters listed in .
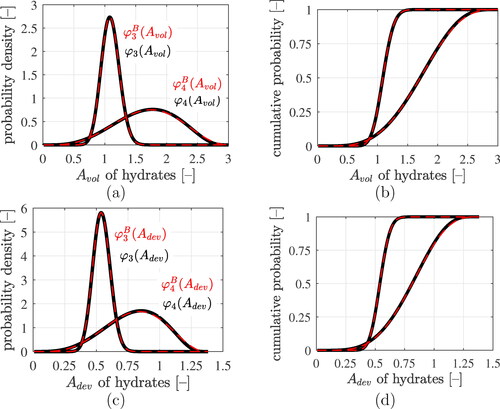
Table 4. Results of the lognormal microelasticity model: (population-averaged) strain concentration tensors components of the four types of solid constituents of Biodentine.
Figure 9. Results of the lognormal microelasticity model (black graphs): statistical distributions of volumetric and deviatoric components of the stress concentration tensors of LDCR hydrates and of HDCR hydrates: (a) and (c) show probability density distributions, (b) and (d) cumulative distribution functions. The best fits of generalized beta-distributions to the statistical distributions are the red dashed graphs, see EquationEqs. (70)(70)
(70) and Equation(71)
(71)
(71) as well as the Beta distributions parameters listed in .
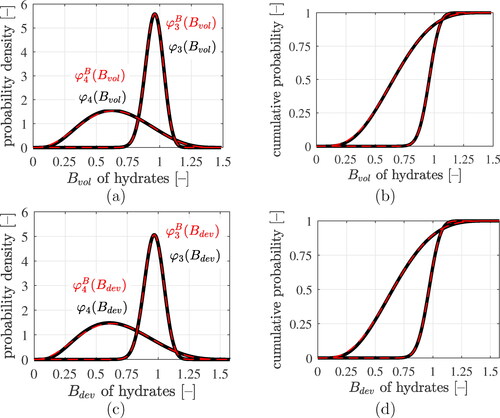
Table 5. Results of the lognormal microelasticity model: (population-averaged) stress concentration tensors components of the four types of solid constituents of Biodentine.
Table 6. Optimal parameters of generalized beta-distributions, see EquationEqs. (70)(70)
(70) and Equation(71)
(71)
(71) , approximating the distributions of the volumetric and deviatoric strain concentration tensor components of both populations of hydrates, see also the dashed red lines in ; R2 denotes coefficients of determination.
Table 7. Optimal parameters of generalized beta-distributions, see EquationEqs. (70)(70)
(70) and Equation(71)
(71)
(71) , approximating the distributions of the volumetric and deviatoric stress concentration tensor components of both populations of hydrates, see also the dashed red lines in ; R2 denotes coefficients of determination.
Figure 10. Micromechanical representation of Biodentine (“material organogram”): the two-dimensional sketch shows qualitative properties of a three-dimensional representative volume element of the piecewise uniform microelasticity models which account for characteristic stiffness constants of two populations of hydrates.
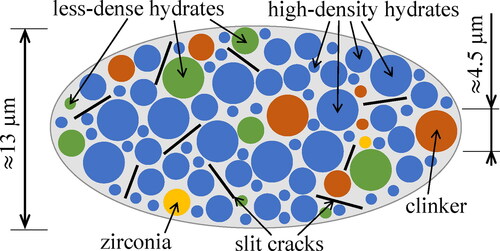
Figure 11. Probability density functions of (a) the bulk and (b) the shear moduli of the LDCR hydrates (red solid graphs) and of the HDCR hydrates (black solid graphs), as well as characteristic stiffness properties: equivalent stiffnesses (dash-dotted lines), mode values (dotted lines), and median values (solid lines).
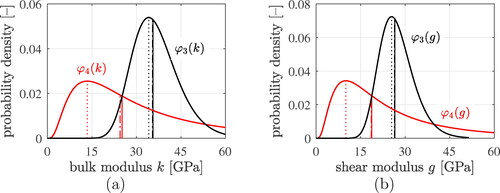
Table 8. Results of the median-based piecewise uniform microelasticity model: strain concentration tensors components of the four types of solid constituents of Biodentine according to EquationEqs. (84)(84)
(84) and Equation(85)
(85)
(85) . The differences of the
and
values to the lognormal model are given in brackets.
Table 9. Results of the median-based piecewise uniform microelasticity model: stress concentration tensors components of the four types of solid constituents of Biodentine according to EquationEqs. (86)(86)
(86) and Equation(87)
(87)
(87) . The differences of the
and
values to the lognormal model are given in brackets.
Figure A.12. Longitudinal and shear wave velocities sent at different frequencies through cylindrical samples of Biodentine, with 5 mm diameter and 10 mm height; the mean longitudinal wave velocity is equal to km/s (upper data points cluster) and the mean shear wave velocity amounts to
km/s (lower data points cluster). The pink markers correspond to 50 kHz transducers central frequency, red to 500 kHz, cyan to 1 MHz, black to 2.25 MHz, green to 5 MHz, blue to 10 MHz, and yellow to 20 MHz transducers’ central frequency, after [3].
![Figure A.12. Longitudinal and shear wave velocities sent at different frequencies through cylindrical samples of Biodentine, with 5 mm diameter and 10 mm height; the mean longitudinal wave velocity is equal to v¯L=4.977 km/s (upper data points cluster) and the mean shear wave velocity amounts to v¯S=2.473 km/s (lower data points cluster). The pink markers correspond to 50 kHz transducers central frequency, red to 500 kHz, cyan to 1 MHz, black to 2.25 MHz, green to 5 MHz, blue to 10 MHz, and yellow to 20 MHz transducers’ central frequency, after [3].](/cms/asset/7cd7a9ad-6ec2-417b-b0a0-f8c49cbb9641/umcm_a_2073493_a0001_c.jpg)
Table A.10. Ultrasonic shear wave transducers used for characterization of hardened Biodentine.