Figures & data
Figure 1. Patient 1. PET/CT in 12/12 (left, prior to vemurafenib) showed innumerable intensely FDG avid lymph nodes and soft tissue deposits scattered throughout the body which developed during her course of ipilimumab by 2 cycles. PET/CT in 2/13 (right, after starting vemurafenib and completion of the ipilimumab course) showed the previously described intensely FDG avid metastases had entirely resolved. The vemurafenib was weaned and completely stopped by 12/13. She has remained in complete remission to date off all therapy.
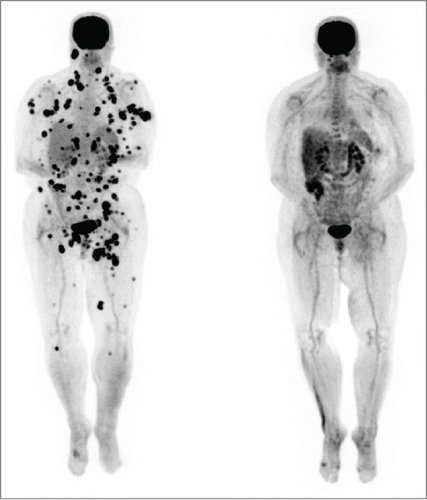
Figure 2. Vitiligo of right arm in Patient 1. The photo shows patchy depigmentation of skin after the patient was treated with vemurafenib but this process had actually started after completion of high dose IL-2.
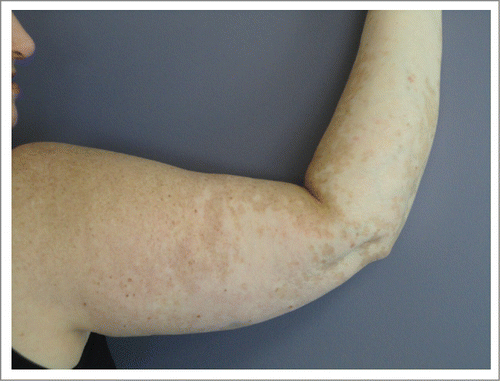
Figure 3. Patient 2. PET/CT on 3/13 (left, prior to vemurafenib) showed intensely FDG avid lymph nodes and soft tissue deposits post ipilimumab. PET/CT on 6/13 (right, after vemurafenib) showed the previously described intensely FDG avid lymph nodes and nodules had entirely resolved. The vemurafenib was gradually weaned and stopped by 3/14. She has remained in complete remission to date off therapy.
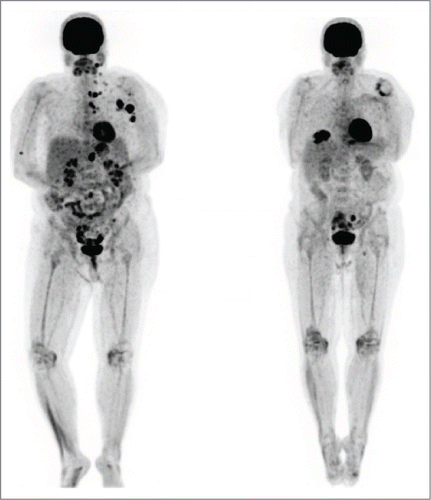
Figure 4. Patient 3. PET/CT in August 2013 (left, prior to BRAF inhibitor therapy) showed intensely FDG avid lymph nodes post ipilimumab which were biopsy confirmed metastatic melanoma. PET/CT in November 2013(right, after trametinib) showed the previously described intensely FDG avid lymph nodes and nodules had entirely resolved. The skin nodules on his scalp also resolved. The trametinib was gradually weaned and stopped by 5/14. He has remained in complete remission to date off therapy.
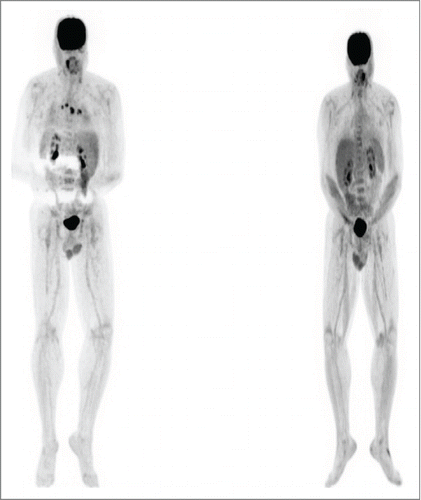
Figure 5. Flow cytometric gating strategy for T-cell phenotyping. Aliquots of freshly collected blood were stained directly with antibodies to the markers shown in A and B. In A, live events were first gated using the FSC × SSC parameters and then the indicated gating strategies were used. In B the lymphocyte fraction was initially gated using the FSC × SSC parameters.
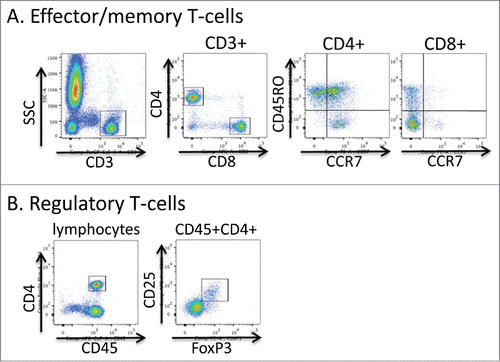
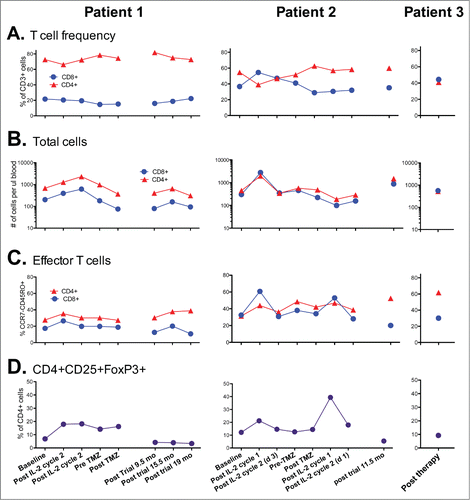
Figure 6. Kinetics of T-cells in the peripheral blood of patients during and following therapy. (A) Ratio of CD4+ and CD8+ T-cells within the CD45+CD3+ cell fraction of fresh blood. (B) Total CD3+CD4+ and CD3+CD8+ T-cells per ul of peripheral blood. (C) Fraction of CD4+ and CD8+ T-cells with the CCR7- CD45RO+ effector phenotype. (D) Frequency of CD4+ cells co-expressing CD25 and FoxP3. Note Patient 3 was not on a prior clinical trial so had no prior baseline determinations for comparison.