Figures & data
Table 1 Patients' characteristics of stage IV CRC patients that were analyzed for presence of baseline CTCs
Table 2 List of the individual patients with CTC counts and tumor/liver volume ratio
Figure 1. Baseline CTC levels correlate with tumor burden in the liver. To statistically analyze the association between CTC numbers and tumor burden in the liver, baseline CTCs were categorized into low (0–2) and high (≥3), and the tumor/liver volume ratio was log-transformed to assure the validity of statistical assumptions. A significant association between the log-transformed tumor/liver volume ratio and baseline low/high CTC levels by both parametric (1two-sample T-test (P = 0.0036)) and non-parametric (Wilcoxon Rank-Sum test) (P = 0.0298) analysis was determined. As shown in , the tumor/liver volume ratio was dichotomized into low (<30%) and high (≥30%) tumor/liver volume ratio, which was also found to be significantly associated with baseline CTC levels (high/low).
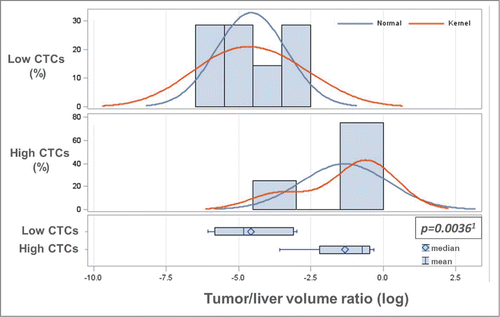
Table 3 Correlation of categorized tumor/liver volume ratio and CEA serum level with baseline CTC numbers in stage IV CRC
Figure 2. Baseline CTC levels correlate with serum CEA levels. Baseline serum CEA levels were log-transformed to stabilize the variance and make the associated statistical assumption more valid. A significant association between log-transformed serum CEA levels and categorized baseline CTC counts (low: 0–2, high: ≥3) was determined by parametric (1two-sample T-test (P = 0.0016)) and non-parametric analysis (Wilcoxon Rank-Sum test (P = 0.0092)). As shown in , baseline serum CEA levels were also categorized and the correlation analysis with low/high baseline CTC levels was also statistically significant.
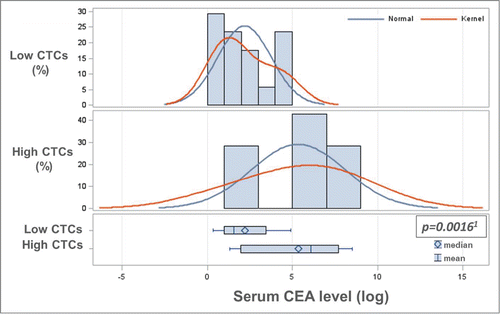
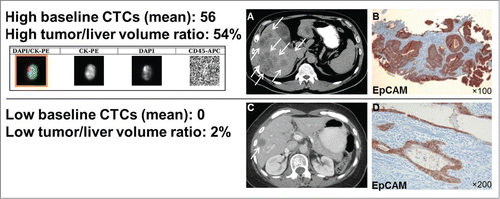
Figure 3. Computer tomography (CT), CTC images and EpCAM immunostaining of CRC tumors. EpCAM is consistently expressed in CRC primary tumors, also in patients that have no CTCs detectable by EpCAM-based CellSearch® system. Shown are: mean CTC numbers (±SEM), the actual liver/tumor volume ratio, a CTC (CellSearch® system qualifies a cell as a CTC if it has an evident nucleus by DAPI and if it is EpCAM+, cytokeratin 8/18/19+, and CD45-), (A) CT scan of a patient with a high tumor/liver ratio (≥30%) and high baseline CTCs (≥3), (B) EpCAM immunostaining with a monoclonal antibody (BerEP4) and peroxidase method of the respective CRC primary tumor, (C) CT scan of a patient with a low high tumor/liver ratio (<30%) zero baseline CTCs, and (D) EpCAM immunostaining of the respective CRC primary tumor.