Figures & data
Figure 1. (A) Metabolism of byproducts of ifosfamide induced toxicity (B) Mechanism of action of evofosfamide.
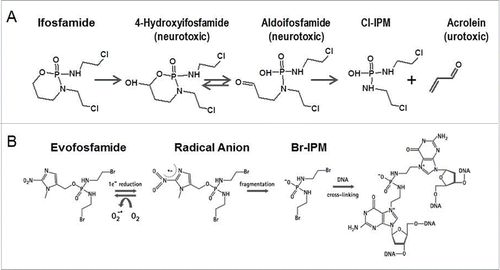
Figure 2. Antitumor activity of evofosfamide or ifosfamide in combination with docetaxel in the metastatic H460 intrapleural model. A, metastasis progression and tumor hypoxia characterization on 4 days, 8 days, and 12 d after H460 cells inoculation. Top panel, H & E staining; middle panel, enlarged images of inserts; and bottom panel, immunofluorescence staining of pimonidazole, a marker of hypoxia, on the consecutive sections of middle panel; green, hypoxia; blue, Hoechst 33342; and red, propidium iodide; B, Kaplan-Meier plot analysis of evofosfamide or ifosfamide as monotherapy. C, Kaplan-Meier plot analysis of evofosfamide or ifosfamide in combination with docetaxel.
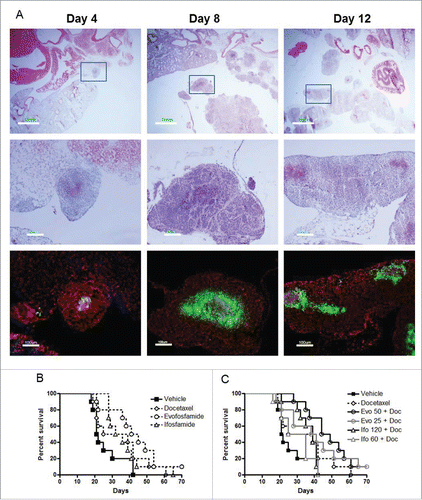
Table 1. Comparison of antitumor activity of evofosfamide and ifosfamide alone, or in combination with docetaxel in the metastatic H460 intrapleural model.
Figure 3. Antitumor efficacy and safety profile of evofosfamide or ifosfamide in combination with docetaxel in the ectopic H460 xenograft model. A and B, tumor growth of evofosfamide or ifosfamide alone (A), or in combination with docetaxel (B). C and D, body weight change induced by evofosfamide or ifosfamide alone (C), or in combination with docetaxel (D). Animals were monitored daily and tumor growth was quantified twice a week. Data are expressed as Mean ± SEM of 10 animals per group. Evo, evofosfamide; Ifo, ifosfamide; Doc, docetaxel.
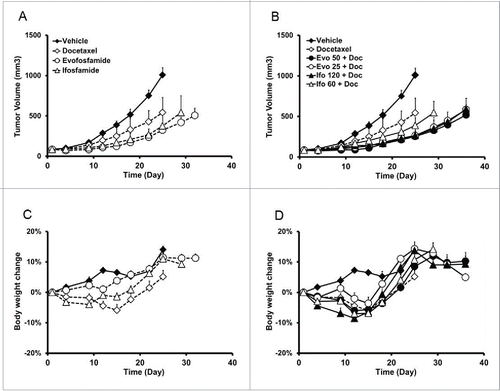
Figure 4. Evofosfamide, but not ifosfamide, exhibits controlled oxygen concentration breathing condition-dependent antitumor activity in the ectopic H460 xenograft model. A and C, antitumor activity; B and D, body weight change as a readout of toxicity. Data are expressed as Mean ± SEM of 10 animals per group. Evo, evofosfamide; Ifo, ifosfamide; Doc, docetaxel. (A and C are from Reference Citation12).
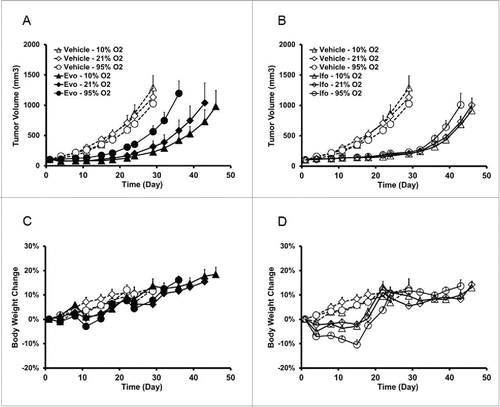
Figure 5. Effect of evofosfamide or ifosfamide on hematologic change in CD1 and H460 tumor bearing nude mice. The means and standard errors from the 5–6 mice per group are presented. A, blood samples were collected 4 hrs after the last treatment from non-tumor bearing CD-1 mice. B, blood samples were collected 3 d after the last treatment from H460 bearing nude mice; *, p < 0.05 as compared to Vehicle.
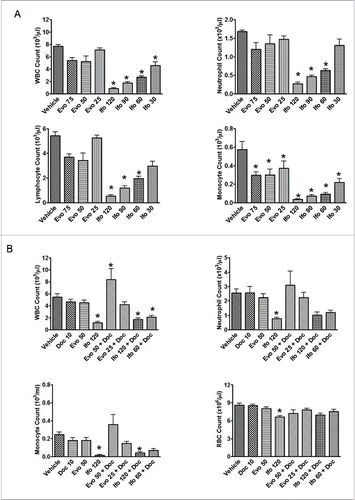
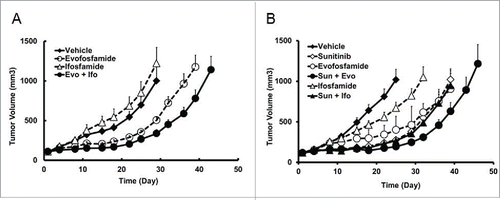
Figure 6. At an equivalent hematoxicity level, antitumor activity of evofosfamide and ifosfamide in the H460 ectopic xenograft models. Evo, was given at 50 mg/kg, ip, and Ifo was given at 30 mg/kg ip at a regimen of QDx5/wk × 2 wks; A, antitumor activity as monotherapy. B, sunitinib was given at 80 mg/kg, QDx19, oral. Evo or Ifo was given 7 d after the initiation of sunitinib treatment. Data are expressed as Mean ± SEM of 10 animals per group.