Figures & data
Figure 1. Mito-CP and Mito-(Q)suppresses viability of B-RafV600E melanoma cells. (A) SK-MEL28, A375, and RPMI-7951 cells in 96-well plates were treated with serially increasing doses of Mito-CP, Mito-Q, or the control compounds, TPP and CP, for 48 h. Cells were then allowed to recover in drug-free fresh medium for 24 h prior to measuring cell viability by MTT assay. Data (mean ± SD, n = 6) are expressed as the percentage of untreated control. (B) A panel of cell lines was treated with 1 µM Mito-CP or Mito-Q for 48 h followed by 24 h recovery prior to MTT assay. Data (mean ± SD, n = 3) are the percentage of untreated control. SK-MEL1, SK-MEL28, A375, and RPMI-7951 (B-RafV600E); SK-MEL 2 (N-RasQ61R); MeWo (B-Raf, N-Ras wild type); BJ and HS27 (primary normal foreskin fibroblasts) (C) Cells of SK-MEL28, A375, and their PLX4032-resistant derivatives were treated with increasing doses of Mito-CP or PLX4032 for 48 h. Cells were then allowed to recover in drug-free fresh medium for 24 h prior to MTT assay.
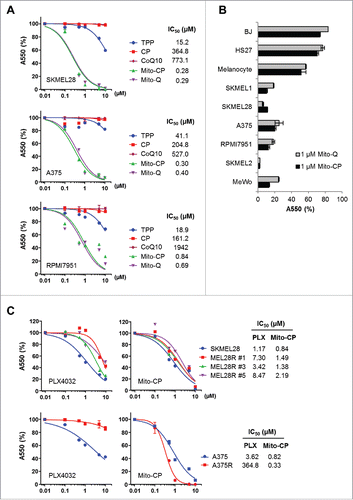
Figure 2. Mito-CP induces surrogate markers of cell death, mitochondrial integrity, and autophagy in B-RafV600E melanoma cells. Total lysates of SK-MEL28, A375, and RPMI-7951 cells treated with different doses of PLX4032 or Mito-CP for 24 h were analyzed by Western blotting for expression of the indicated proteins. β-actin is the control for equal amounts of protein loading.
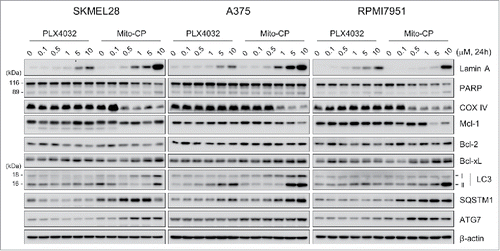
Figure 3. Mito-CP induces apoptotic cell death. SK-MEL28 (A) and A375 (B) cells were treated with increasing doses of Mito-CP for 24 h prior to annexin V/propidium iodide staining. The graphs (right) indicate annexin V positive and propidium iodide positive cell populations. 50 µM cisplatin treatment for 24 h was used as a positive control to induce apoptosis. Cisplatin was dissolved in water. Data (mean ± SEM) are from a representative experiment performed in triplicate. *P < 0.05; ***P < 0.001.
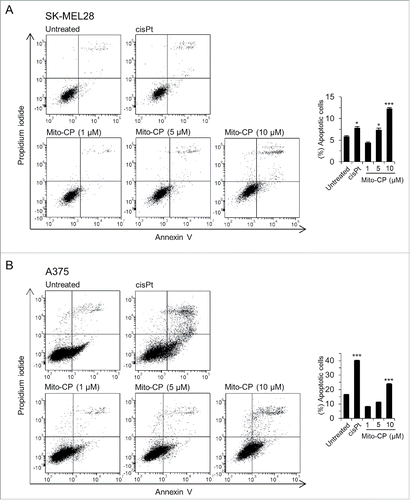
Figure 4. Mito-CP induces loss of mitochondrial membrane potential in SK-MEL28 cells. (A) Cells treated with 5 µM Mito-CP for 1 h were stained with TMRE. Changes in the mitochondrial membrane potential were then visualized under a fluorescent microscope (magnification 400X). MitoTracker Green was used to visualize intact mitochondria in cells. PBS was used as the control for Mito-CP. (B) Cells treated with Mito-CP or PLX4032 for 24 h were stained with TMRE and were analyzed by flow cytometry to measure red fluorescence (PE channel, 575 nm). Unstained cells were used as negative controls. PBS and DMSO are vehicle controls for Mito-CP and PLX4032, respectively. (C) Cytosolic and mitochondrial extracts from 5 × 105 cells treated with Mito-CP for 18 h were analyzed by Western blotting for TPP adduct formation. COX IV and β-actin were detected as the controls for purity of extracts. (D) Cells treated with Mito-CP for 24 h were treated with 1 μM carboxy-H2DCFDA for 1 h and then incubated for 2 h in a dye-free culture medium. Cells were harvested and analyzed by flow cytometry to measure green fluorescence (FITC channel, 525 nm).
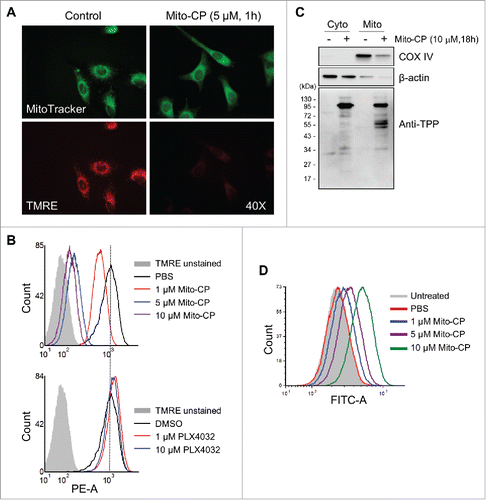
Figure 5. The ROS scavenger, NAC, inhibits Mito-CP-induced death in B-RafV600E melanoma cells. SK-MEL28 and A375 cells were pretreated with different concentrations of NAC for 1 h prior to 10 μM Mito-CP treatment for 18 h. Cells were then subject to Western blot analysis (A) and trypan blue exclusion cell scoring (B). Data (mean ± SEM) are from a representative experiment conducted in triplicate. Control cells were treated with equal volume of PBS. *P value < 0.05.
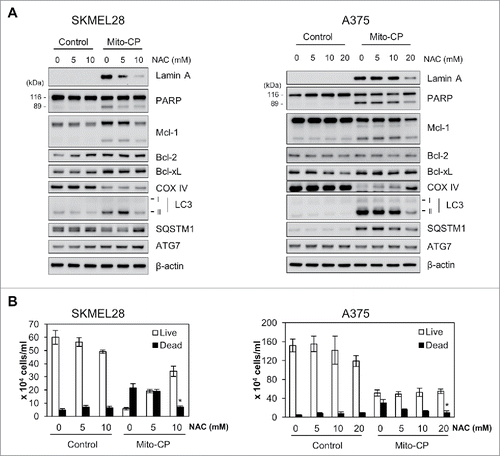
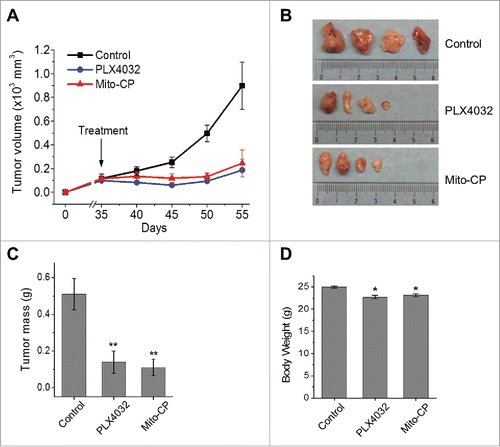
Figure 6. Mito-CP suppresses SK-MEL28 xenografts in athymic mice. Athymic mice bearing SK-MEL28 xenografts were treated with PLX4032 (50 mg/kg/dose) or Mito-CP (50 mg/kg/dose). Drugs dissolved in 200 μl vehicle were administered per os beginning from day 35 after tumor implantation. The control group was treated with the vehicle only (details in the Materials and Methods). (A) Changes in tumor sizes were determined at the indicated time-points. (B) Images of tumors collected at the end of the treatment. (C) Weights of tumors collected at the end of the treatment. (D) Body weights of animals at the end of the treatment. All data are mean ± SEM, n = 4, *P value < 0.05, **P value < 0.01.