Figures & data
Figure 1. Structures of rabbit, human, and rabbit-human chimeric antibodies. PDB file information is indicated below each structure.Citation13-15
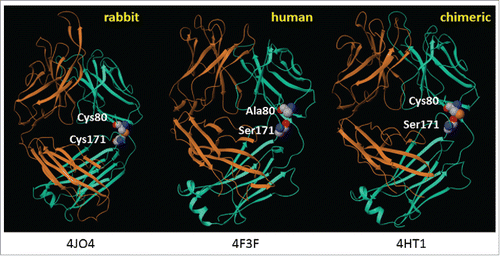
Figure 2. Rabbit-human chimeric 155D5 is cysteinylated at C80. Rabbit-human chimeric anti-human CA9 mAb 155D5 was purified using protein A affinity chromatography, reduced using harsh (20 mM DTT, 60°C, panel (A) or very mild (100 µM DTT, room temperature, panel (B) conditions and analyzed by mass spectrometry. The mass shift of 120 Da indicates the presence of a capping cysteine group on the light chain.
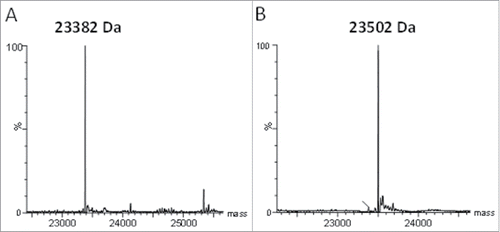
Figure 3. The cysteinylation on C80 can be removed by mild reducing conditions. Mass spectrometry analysis of deglycosylated (panel A) and deglycosylated/decysteinylated (panel B) xi155D5. The observed mass of 145236 Da matches the predicted mass of the deglycosylated/decysteinylated mAb.
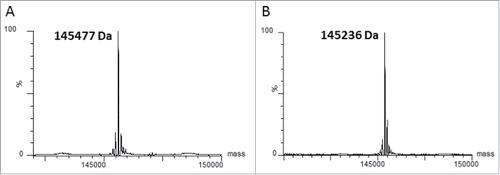
Figure 4. A decysteinylated C80 can be efficiently conjugated to maleimides with no impact on antigen binding affinity (A) Mass spectrometry analyses of the light chain of decysteinylated xi155D5 conjugated with maleimido-PEG2-biotin. Efficiency of conjugation was 94%. (B). Mass spectrometry analyses of the light chain of decysteinylated xi155D5, xi155D5 conjugated with maleimido-PEG4- DBCO, and xi155D5-DBCO conjugated with azido-chlorothiazide via SPAAC. Efficiency of maleimido addition to the light chain was 85%. SPR analysis of the binding affinity of cysteinylated, decysteinylated, and decysteinylated/conjugated mAb to soluble CA9 indicated that the decysteinylation procedure as well as subsequent conjugation to C80 had no impact on binding affinity of the xi155D5 antibody to antigen.
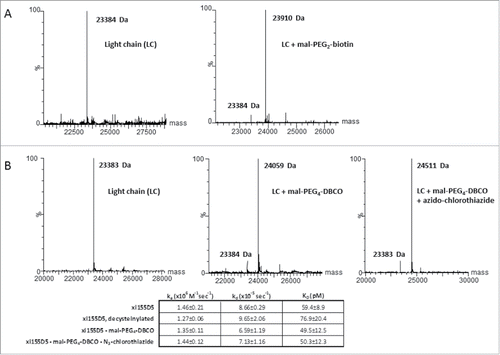
Figure 5. Tumor-specific localization of anti-human CA9 xi155D5–800CW. Human colo205 (upper panel) and HT-29 (lower panel) cells were grafted into nude mice, which were subsequently injected with xi155D5–800CW. Fluorescent signal (orange-red) was monitored at various time point (shown are 0–72 hours and right flank). Approximate location of kidney (K) and tumor (T) is indicated.
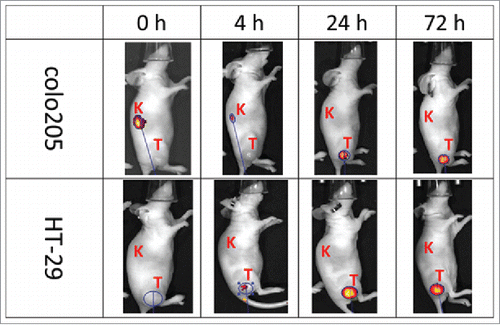
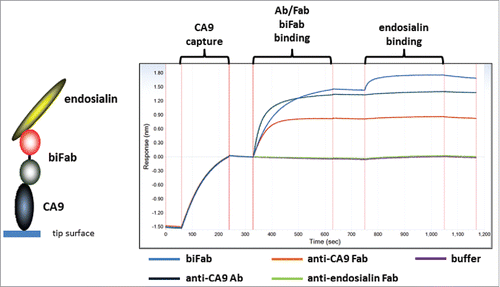
Figure 6. In vivo specificity and efficacy of rabbit-human chimeric anti-human mesothelin – auristatin F C80 ADCs. (A) Schematic of conjugation of maleimido-PEG2-auristatin F to C80. (B) Anti-human mesothelin binding, aggregation, and ADC cytotoxicity data. Affinities are shown pre- and post-conjugation. (C) In vivo efficacy of anti-mesothelin auristatin F ADCs. Anti-human endosialin xi1–55–2 – AuF was dosed similarly to anti-mesothelin ADCs.
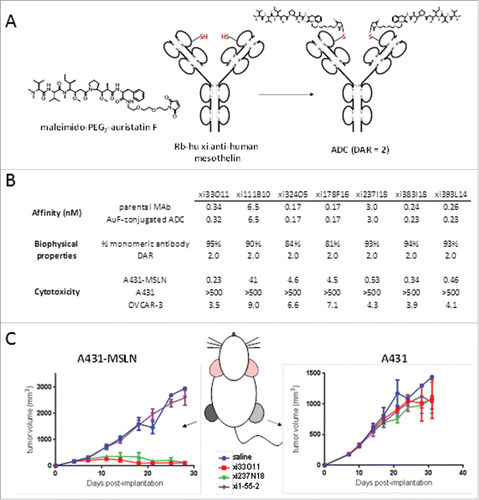
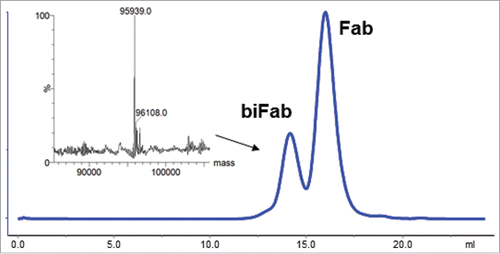
Figure 7. Purification of biFab heterodimer. Following overnight incubation of DBCO- and azido-conjugated Fabs, RESPECT bispecific antibody (biFab) was purified by S-200 SEC chromatography. The fractions containing the RESPECT bispecific antibody were pooled and the mass was analyzed by mass spectrometry (inset).