Figures & data
Figure 1. Treatment of NSCLC cells and ovarian cancer cells with [pemetrexed + sildenafil] reduces the protein expression of multiple histone deacetylase proteins. A. NSCLC cells and ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of HDACs1–11 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells. B. NSCLC cells were transfected with a scrambled siRNA or siRNA molecules to knock down the expression of the AMPK α subunit, ATG5 or Beclin1. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunostaining performed to determine the expression of HDAC6 (n = 3 +/−SEM). *p < 0.05 lower than corresponding values in cells with knock down of AMPK, ATG5 or Beclin1; #p < 0.05 greater than corresponding vehicle control value. C. The PDX NSCLC isolate ADOR was transfected with a control siRNA or with an siRNA to knock down Beclin1 expression. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then fixed in place and the expression of the HDAC proteins determined by immunostaining. (n = 3 +/−SEM) * p < 0.05 less than corresponding intensity in siSCR cells.
![Figure 1. Treatment of NSCLC cells and ovarian cancer cells with [pemetrexed + sildenafil] reduces the protein expression of multiple histone deacetylase proteins. A. NSCLC cells and ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of HDACs1–11 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells. B. NSCLC cells were transfected with a scrambled siRNA or siRNA molecules to knock down the expression of the AMPK α subunit, ATG5 or Beclin1. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunostaining performed to determine the expression of HDAC6 (n = 3 +/−SEM). *p < 0.05 lower than corresponding values in cells with knock down of AMPK, ATG5 or Beclin1; #p < 0.05 greater than corresponding vehicle control value. C. The PDX NSCLC isolate ADOR was transfected with a control siRNA or with an siRNA to knock down Beclin1 expression. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then fixed in place and the expression of the HDAC proteins determined by immunostaining. (n = 3 +/−SEM) * p < 0.05 less than corresponding intensity in siSCR cells.](/cms/asset/0eb21e4a-3226-4d28-9bc3-d18ec0ce3b89/kcbt_a_1362511_f0001_b.gif)
Figure 2. [Pemetrexed + sildenafil] lethality is enhanced by HDAC inhibitors. A. NSCLC cells were treated with vehicle control, [pemetrexed (1 μM) + sildenafil (2 μM)], sodium valproate (250 μM) or together in the indicated 3 drug combinations. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value. B. A549 cells were transfected with an siRNA control (siSCR) or siRNA molecules to knock down HDACs 1/2/3/6/8/10. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)]. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value; ## p < 0.05 greater than corresponding value in HDAC6 knock down cells.
![Figure 2. [Pemetrexed + sildenafil] lethality is enhanced by HDAC inhibitors. A. NSCLC cells were treated with vehicle control, [pemetrexed (1 μM) + sildenafil (2 μM)], sodium valproate (250 μM) or together in the indicated 3 drug combinations. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value. B. A549 cells were transfected with an siRNA control (siSCR) or siRNA molecules to knock down HDACs 1/2/3/6/8/10. Twenty-four h after transfection cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)]. Cells were isolated after 12h and live/dead assays performed to determine the percentage cell death (n = 3 +/−SEM). # p < 0.05 greater than [pemetrexed + sildenafil] value; ## p < 0.05 greater than corresponding value in HDAC6 knock down cells.](/cms/asset/9b7403d3-9b8d-4557-bf1f-794ba1ba6ef9/kcbt_a_1362511_f0002_b.gif)
Figure 3. Pemetrexed and sildenafil interact to enhance ceramide levels via CerS6 in a nitric oxide-dependent fashion. A. A549 NSCLC cells (1 × 106) were transfected with an empty vector plasmid (CMV) or with a plasmid to express thioredoxin (TRX). Twenty-four h after transfection cells were treated with vehicle control or L-NAME (10 μM). Thirty minutes later cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then harvested in 550 µl of cold PBS and 50 µl taken for lysis and protein determination using the Bradford Assay (Bio-Rad). Sphingolipid levels were normalized based on total protein levels for each sample. Cells were processed and subjected to quantitative mass spectrometry to determine the levels of sphingolipid species (n = 3 +/−SEM). #p < 0.05 greater than corresponding vehicle control; *p < 0.05 less than corresponding value in CMV transfected cells. B. NSCLC cells were transfected with a scrambled control (siSCR) or with various validated siRNA molecules to knock down the expression of the indicated histone deacetylase (HDAC) proteins. Twenty-four h after transfection, cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater fluorescence than siSCR control; * p < 0.05 less fluorescence than siSCR control. C. A549, H460, H1975 and ADOR cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h and 12h. The mean number of intense GFP+ and RFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). The data for each bar is the mean level of GFP+/RFP+ vesicles collectively from all 4 cell lines. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of Beclin1. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. After 6h cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater than vehicle control.
![Figure 3. Pemetrexed and sildenafil interact to enhance ceramide levels via CerS6 in a nitric oxide-dependent fashion. A. A549 NSCLC cells (1 × 106) were transfected with an empty vector plasmid (CMV) or with a plasmid to express thioredoxin (TRX). Twenty-four h after transfection cells were treated with vehicle control or L-NAME (10 μM). Thirty minutes later cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were then harvested in 550 µl of cold PBS and 50 µl taken for lysis and protein determination using the Bradford Assay (Bio-Rad). Sphingolipid levels were normalized based on total protein levels for each sample. Cells were processed and subjected to quantitative mass spectrometry to determine the levels of sphingolipid species (n = 3 +/−SEM). #p < 0.05 greater than corresponding vehicle control; *p < 0.05 less than corresponding value in CMV transfected cells. B. NSCLC cells were transfected with a scrambled control (siSCR) or with various validated siRNA molecules to knock down the expression of the indicated histone deacetylase (HDAC) proteins. Twenty-four h after transfection, cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater fluorescence than siSCR control; * p < 0.05 less fluorescence than siSCR control. C. A549, H460, H1975 and ADOR cells were transfected with a plasmid to express LC3-GFP-RFP. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h and 12h. The mean number of intense GFP+ and RFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). The data for each bar is the mean level of GFP+/RFP+ vesicles collectively from all 4 cell lines. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of Beclin1. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. After 6h cells were fixed in place and the expression of CerS6 determined (n = 3 +/−SEM). # p < 0.05 greater than vehicle control.](/cms/asset/4835e2d7-e8f0-4dee-af2c-5b4924a90d24/kcbt_a_1362511_f0003_b.gif)
Figure 4. Nitric oxide generation and ceramide generation cooperate to cause drug-induced CD95 tyrosine phosphorylation, plasma membrane localization and DISC formation. A. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. Portions of the cells are fixed in place without permeabilization. Immunostaining is performed to detect the total membrane levels of CD95 (n = 3 +/−SEM). B. Another portion of the drug-treated cells from Panel A. is lysed and subjected to immunoprecipitation for CD95. The association of FADD and caspase 8 with CD95 is determined after SDS PAGE and immunoblotting. C. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are pre-treated for 30 min with vehicle, N-acetyl cysteine (10 μM) or L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. The cells are lysed and subjected to immunoprecipitation for CD95. The total expression and tyrosine phosphorylation of CD95 is determined after SDS PAGE and immunoblotting. The bar graph presents the mean-fold alterations in CD95 tyrosine phosphorylation from all cell lines tested under each condition (n = 3 +/− SEM). * p < 0.05 less than corresponding value in [siSCR + vehicle] cells. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of PTEN. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. CD95 was immunoprecipitated and the tyrosine phosphatase activity associated with CD95 measured as described in the Methods. Immunoprecipitates were then subjected to SDS PAGE and immunoblotting to determine the levels of PTPN13 in each precipitate (n = 3 +/−SEM).
![Figure 4. Nitric oxide generation and ceramide generation cooperate to cause drug-induced CD95 tyrosine phosphorylation, plasma membrane localization and DISC formation. A. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. Portions of the cells are fixed in place without permeabilization. Immunostaining is performed to detect the total membrane levels of CD95 (n = 3 +/−SEM). B. Another portion of the drug-treated cells from Panel A. is lysed and subjected to immunoprecipitation for CD95. The association of FADD and caspase 8 with CD95 is determined after SDS PAGE and immunoblotting. C. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are pre-treated for 30 min with vehicle, N-acetyl cysteine (10 μM) or L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. The cells are lysed and subjected to immunoprecipitation for CD95. The total expression and tyrosine phosphorylation of CD95 is determined after SDS PAGE and immunoblotting. The bar graph presents the mean-fold alterations in CD95 tyrosine phosphorylation from all cell lines tested under each condition (n = 3 +/− SEM). * p < 0.05 less than corresponding value in [siSCR + vehicle] cells. D. Cells were transfected with a scrambled control siRNA or an siRNA to knock down expression of PTEN. Twenty-four h after transfection cells were pre-treated with vehicle control or with L-NAME (1 μM), and then treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 3h. CD95 was immunoprecipitated and the tyrosine phosphatase activity associated with CD95 measured as described in the Methods. Immunoprecipitates were then subjected to SDS PAGE and immunoblotting to determine the levels of PTPN13 in each precipitate (n = 3 +/−SEM).](/cms/asset/c6104614-63cb-4843-8853-5e27f645fe8f/kcbt_a_1362511_f0004_b.gif)
Figure 5. [Pemetrexed + sildenafil] -induced autophagosome and autolysosome formation requires CerS6 expression. A. ADOR NSCLC cells were transfected with a plasmid to express LC3-GFP and in parallel transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. The mean number of intense GFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). B. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 24h. Cell viability was determined by live/dead assay (n = 3 +/−SEM).
![Figure 5. [Pemetrexed + sildenafil] -induced autophagosome and autolysosome formation requires CerS6 expression. A. ADOR NSCLC cells were transfected with a plasmid to express LC3-GFP and in parallel transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. The mean number of intense GFP+ vesicles staining under each condition is determined (n = 40 cells per condition +/− SEM). B. NSCLC cells were transfected with a scrambled siRNA (siSCR) or with an siRNA to knock down CerS6 expression. Twenty-four h after transfection cells are treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 24h. Cell viability was determined by live/dead assay (n = 3 +/−SEM).](/cms/asset/705f332c-3605-46cc-a3a8-eb82c9901af6/kcbt_a_1362511_f0005_b.gif)
Figure 6. Exposure of cells to [pemetrexed + sildenafil] reduces the expression of PD-L1, PD-L2 and ODC and increases the expression of MHCA. A: NSCLC cells; B: in vivo generated afatinib resistant H1975 clones; and C: ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC and the expression and localization of HMGB1 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells; #p < 0.05 significantly greater staining intensity than that in vehicle control treated cells.
![Figure 6. Exposure of cells to [pemetrexed + sildenafil] reduces the expression of PD-L1, PD-L2 and ODC and increases the expression of MHCA. A: NSCLC cells; B: in vivo generated afatinib resistant H1975 clones; and C: ovarian cancer cells were treated with vehicle control or with [pemetrexed (1 μM) + sildenafil (2 μM)] for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC and the expression and localization of HMGB1 (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in vehicle control treated cells; #p < 0.05 significantly greater staining intensity than that in vehicle control treated cells.](/cms/asset/3b2b404c-6484-4e98-9dbc-00aed9d4fb7b/kcbt_a_1362511_f0006_b.gif)
Figure 7. Afatinib-resistant H1975 cells express higher levels of HDAC3, HDAC10 and ODC and lower levels of MHCA; knock down of HDACs 3+10 increases MHCA expression and reduces ODC expression. A. H1975 cells (wild type clones and afatinib resistant clones) were fixed in place and the expression of HDACs1–11 determined by immuno-fluorescence. HDACs for whom their expression changed are presented (HDACS3/4/5/7/10). The mean fluorescence intensity from 40 cells in each wild type and afatinib resistant clone was determined and the -Fold change between wild type and afatinib resistant clones determined (n = 3 +/− SEM). # p < 0.05 greater than value in wild type clones; * p < 0.05 less than value in wild type clones. B. H1975 cells (wild type clones and afatinib resistant clones) were fixed in place and the expression of PD-L1, PD-L2, MHCA, ODC, HMGB1 and HSP70 determined by immuno-fluorescence. The mean fluorescence intensity from 40 cells in each wild type and afatinib resistant clone was determined and the -Fold change between wild type and afatinib resistant clones determined (n = 3 +/− SEM). # p < 0.05 greater than value in wild type clones; * p < 0.05 less than value in wild type clones. C. Afatinib resistant H1975 clones were treated with vehicle control or with sodium valproate (250 μM) for 6h. Cells were fixed in place and the expression of PD-L1, PD-L2, MHCA, and ODC determined by immuno-fluorescence. The mean fluorescence intensity from 40 cells in each vehicle treated and valproate treated clone was determined and the -Fold change between vehicle and valproate determined (n = 3 +/− SEM). # p < 0.05 greater than value in vehicle; * p < 0.05 less than value in vehicle. D. Afatinib resistant H1975 clones were scramble control transfected or transfected to knock down expression of HDAC3, HDAC10 or both HDACs together. Twenty-four h after transfection cells were fixed in place and immuno-fluorescence performed to determine the expression of PD-L1, PD-L2, MHCA and ODC. The mean fluorescence intensity from 40 cells in each condition was determined and the -Fold change between vehicle and valproate determined (n = 3 +/− SEM). # p < 0.05 greater than value in siSCR; * p < 0.05 less than value in siSCR; ** p < 0.05 less than value in siHDAC10 alone.
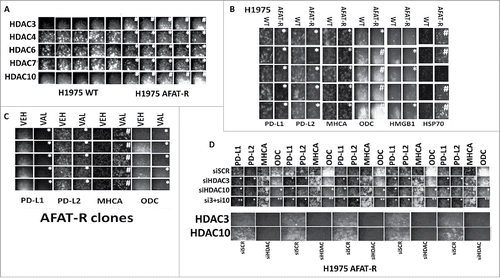
Figure 8. [Pemetrexed + sildenafil + valproate] kills lung cancer cells and facilitates checkpoint inhibitor immunotherapy anti-tumor effects. A. Lewis Lung Carcinoma cells were treated with vehicle control, pemetrexed (1.0 μM), sildenafil (2.0 μM), sodium valproate (250 μM), or the drugs in combination as indicated for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in pemetrexed treated cells; #p < 0.05 significantly greater staining intensity than that in pemetrexed treated cells. ¶ p < 0.05 significantly lower than in vehicle control treated cells. ** p < 0.05 less than value in [pemetrexed + sildenafil] treated cells. ## p < 0.05 greater than value in [pemetrexed + sildenafil] treated cells. B. and C. Lewis Lung Carcinoma cells were grown in C57 black mice and animals were treated with vehicle control, sodium valproate, [pemetrexed + sildenafil], [pemetrexed + sildenafil + sodium valproate] in the presence of a control IgG, and anti-PD-1 IgG or an anti-CTLA4 IgG as described in the Methods. (n = 10 per group +/−SEM). * p < 0.05 lower tumor growth than vehicle treated tumors; ** p < 0.05 lower growth than [pemetrexed + sildenafil] treated tumors; ¶ p < 0.05 less that [pemetrexed + sildenafil] + control IgG.
![Figure 8. [Pemetrexed + sildenafil + valproate] kills lung cancer cells and facilitates checkpoint inhibitor immunotherapy anti-tumor effects. A. Lewis Lung Carcinoma cells were treated with vehicle control, pemetrexed (1.0 μM), sildenafil (2.0 μM), sodium valproate (250 μM), or the drugs in combination as indicated for 6h. Cells were fixed in place and immunofluorescence staining performed to detect the protein expression levels of PD-L1, PD-L2, MHCA, ODC (n = 3 +/−SEM) *p < 0.05 significantly lower staining intensity than that in pemetrexed treated cells; #p < 0.05 significantly greater staining intensity than that in pemetrexed treated cells. ¶ p < 0.05 significantly lower than in vehicle control treated cells. ** p < 0.05 less than value in [pemetrexed + sildenafil] treated cells. ## p < 0.05 greater than value in [pemetrexed + sildenafil] treated cells. B. and C. Lewis Lung Carcinoma cells were grown in C57 black mice and animals were treated with vehicle control, sodium valproate, [pemetrexed + sildenafil], [pemetrexed + sildenafil + sodium valproate] in the presence of a control IgG, and anti-PD-1 IgG or an anti-CTLA4 IgG as described in the Methods. (n = 10 per group +/−SEM). * p < 0.05 lower tumor growth than vehicle treated tumors; ** p < 0.05 lower growth than [pemetrexed + sildenafil] treated tumors; ¶ p < 0.05 less that [pemetrexed + sildenafil] + control IgG.](/cms/asset/15ac4702-ffa8-4099-a006-e75febf46c89/kcbt_a_1362511_f0008_b.gif)
