Figures & data
Figure 1. The VASH1 gene is located on chromosome14q24.3 and contains eight exons with 5589 bp. The primary product of VASH1 was named VASH1A, and contains 365 amino and 44 kDa. However, only 42 kDa have been detected and exhibit antiangionenic activities. VASH1B contains five exons lacking exon 6–8, with 1459 bp and 204 aa. The VASH2 gene is located on 1q32.3, and contains 1–11 exons. The major transcript contains exons 1/2/4/5/6/8/9/11, with 3589 bp and 355 aa
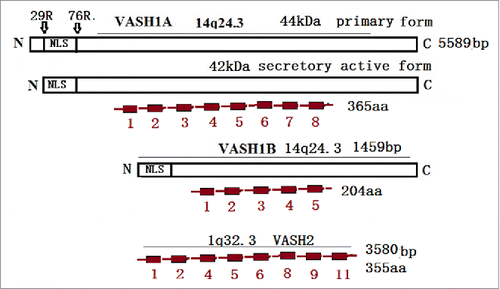
Figure 2. The VASH1 is expressed in the termination zone of vessels in order to stop angiogenesis. TNFα, TL-1β, and IFNγ stop the role of VEGF induction. The VASH2 is mainly presented at the sprout zone of vessels in order to promote angiogenesis. Under hypoxic conditions, the VEGF is up-regulated by HIF-1α. Also, VASH1 increases the PHD levels, which promotes the degradation of HIF-1α
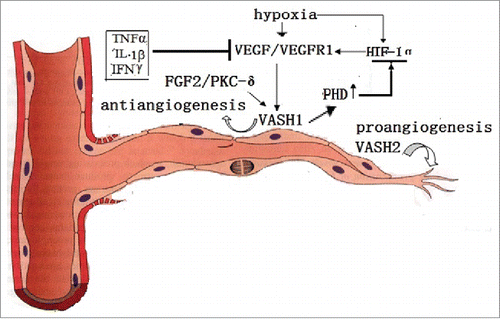
Figure 3. ROS and stress induces the death or damage of endothelial cells. The endogenous VASH1 improves the stress tolerance of endothelial cells to ROS and stress by up-regulating the expression of SOD2 and SIRT1. Also, HuR modulates both the transcription and post transcription of the VASH1
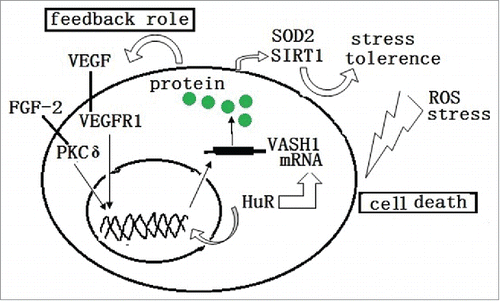
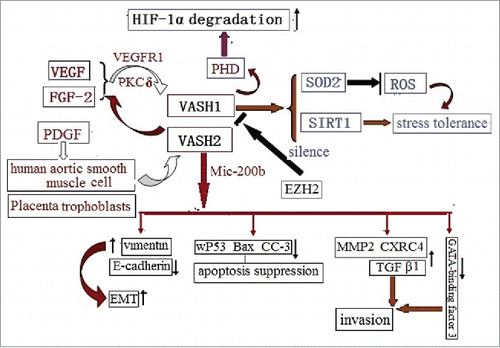
Figure 4. The VASH2 is released by both human aortic smooth muscle cells, and placenta trophoblasts, via PDGF. In a vice-versa manner, it decreases the release of VEGF and FGF2. The VASH2 is silenced by EZH2, and is trans-activated by Mic-200b, in order to promote EMT via vimentin up-regulation and E-cadherin down-regulation. This results in decreased apoptosis via the down-regulation of the wild-type of p53, Bax, and CCP-3; promotes invasions via the up-regulation of MMP2, CXRC4, and TGFβ1; and regresses the GATA-binding factor.