Figures & data
Figure 1. Overexpression of HN1L associated with poorer outcomes of NSCLCs. (a) Relative expression levels of HN1L detected by qPCR in 45 pairs of NSCLC tissues. (b) Western blot analysis of HN1L expression in matched primary tumor (T) samples and its corresponding non-tumor (N) tissues of 8 NSCLC cases. β-Tubulin was used as a loading control. (c) Representative IHC staining figures of HN1L expression in lung squamous cell carcinoma and adenocarcinoma. Scale bar, top figures, 100 μm; bottom figures, 50μm. (d) Relative expression levels of HN1L detected by qPCR and western blotting in an immortalized bronchial epithelial cell line (BEAS-2B) and other five NSCLC cell lines. Data represent mean ± SD. * P < 0.05. β-Actin was used as a loading control. (e) Kaplan-Meier analysis indicating the correlation of HN1L overexpression with poorer overall survival and disease-free survival rates of NSCLC patients.
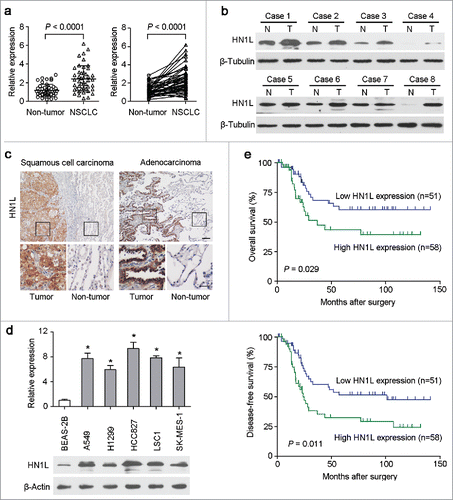
Table 1. Correlation of HN1L expression with clinicopathological features in NSCLC patients.
Table 2. Univariate and multivariate analyses of overall survival in NSCLC patients.
Figure 2. Knockdown of HN1L by shRNA supressed its malignancy in vitro and in vivo. (a) One shRNA against HN1L (sh-HN1L) effectively decreased HN1L expression as detected by western blotting. Scrambled shRNA (sh-control)-transfected cells and parental cells were used as negative controls. β-Tubulin was used as a loading control. Silencing HN1L expression could effectively inhibit cell growth (b), foci formation efficiencies (b), spheres formation in soft agar (d) in vitro. (e) The xenograft tumor weights were measured after surgery (n = 9). (f) Representative IHC images of HN1L expression in xenograft tumors. Scale bar, 100 μm. In all panels, data represent mean ± SD. *P < 0.05.
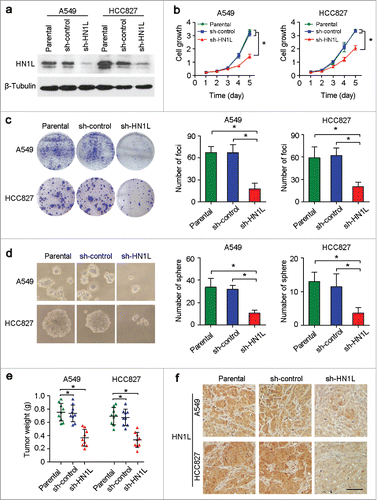
Figure 3. Aberrant HN1L expression indicated the malignant proliferation. (a) Knockdown of HN1L inhibited cell proliferation measured by BrdU incorporation assay. Scale bar, 50 μm. Data represent mean ± SD. *P < 0.05. (b) IHC staining showed the low-proportion of proliferative cells in tumor produced from HN1L silenced cells. Scale bar, 50 μm. Data represent mean ± SD. *P < 0.05. (c) The IC50 values of paclitaxel and cisplatin for A549 and HCC827 cells with or without HN1L silencing. Data represent mean ± SEM. *P < 0.05.
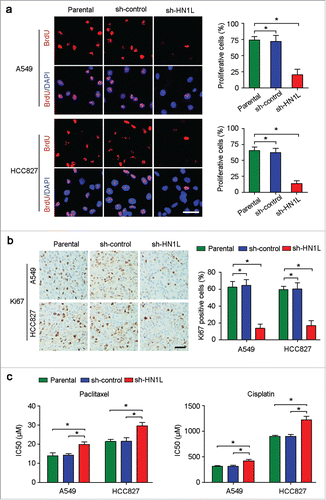
Table 3. Correlation between HN1L and Ki67 expression in NSCLC patients.
Figure 4. Silencing HN1L caused cell cycle arrest by interfering MAPK pathway. (a) Cell cycle distributions were analyzed by flow cytometry. *P < 0.05 (Chi-squared test). (b) Western blot analyzed the protein levels of several cell cycle regulators in sh-HN1L, sh-control and parental cells. β-Tubulin was used as a loading control. (c) Representative IHC images of p21 and p53 expressions in xenograft tumors. Scale bar, 150 μm. (d) Western blot analysis was applied to detect the phosphorylation levels of the key mediators in MAPK pathway (phospho-c-Raf(Ser259), phospho-MEK1/2(Ser217/221), phospho-Erk1/2(Thr202/204), phospho-p90RSK(Ser380) and phospho-Elk-1(Ser383)). β-Tubulin was used as a loading control. (e) The phosphorylation levels of Akt(Thr308/Ser473) was tested by western blotting. β-Actin was used as a loading control.
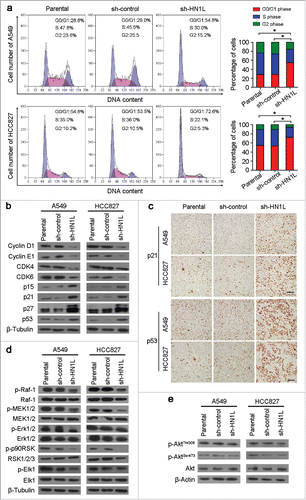
Figure 5. HN1L regulates Ras/MAPK pathway via interacting with RASA4. (a) List of the top HN1L-interacting proteins from the public databases. Co-immunoprecipitation (b) and immunofluorescent double-staining (c) indicate the direct interaction of HN1L and RASA4 in A549 and HCC827 cells. (d) GST-Raf1-RBD fusion protein is used to bind the activated form of GTP-bound Ras, which can then be immunoprecipitated with glutathione resin. Ras activation levels in A549 and HCC827 cells with or without HN1L silencing are then determined in western blot using a Ras monoclonal antibody. (e) Immunofluorescent staining showed the reduction of membrane localization of Raf-1 after knockdown of HN1L. Scale bar, 10 μm.
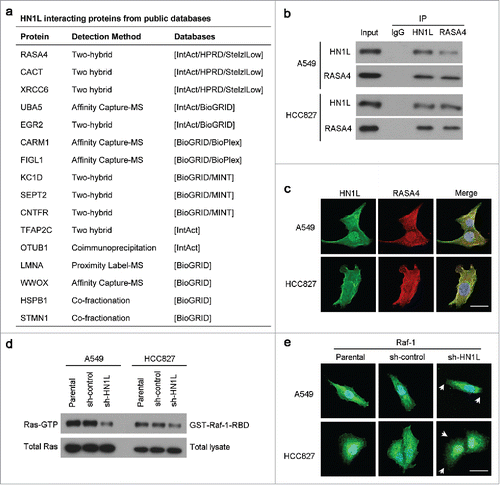
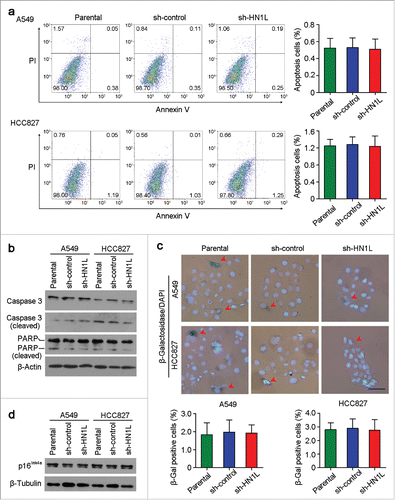
Figure 6. Knockdown of HN1L could not induce cell apoptosis and senescence. (a) Apoptosis cells were analyzed by flow cytometry (Annexin V-FITC/PI staining). Data represent mean ± SD. (b) Western blot analyzed the levels of several apoptosis associated proteins in sh-HN1L, sh-control and parental cells. β-Actin was used as a loading control. (c) Cell senescence was tested by β-Galactosidase (β-Gal) staining. Representative β-Gal positve cells were indicaed by red arrows. Scale bar, 50 μm. Data represent mean ± SD. (d) Western blotting showed the protein level of p16Ink4a in A549 and HCC827 cells before or after HN1L silencing. β-Tubulin was used as a loading control.