Figures & data
Table 1. Sarcoma patients' clinic-pathological characteristics.
Table 2. Patients' treatment, outcome and CTCs counting and characterization.
Figure 1. A) Negative control, A-549 cell line “spiked” in healthy blood and negative for EGFR. B) Positive control, FaDu cell line “spiked” in healthy blood and stained for EGFR. C, D) Examples of an isolated CTC of sarcoma patient with cytomorphological features (negative staining for CD45, nucleus size ≥ 12 µm, hyperchromatic and irregular nucleus, visible presence of cytoplasm, and a high nucleus–cytoplasm ratio (Krebs, et al., 2012)Citation15. E) Immunocytochemistry of CTC with anti-EGFR antibody and counterstaining with DAB. F) One CTM from STS patient observed in the blood filtered using the ISET.
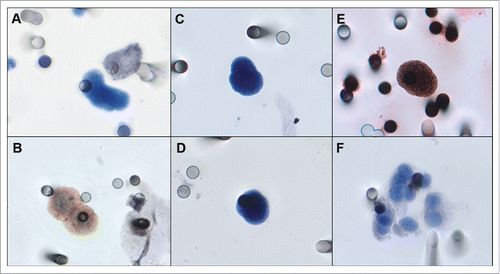
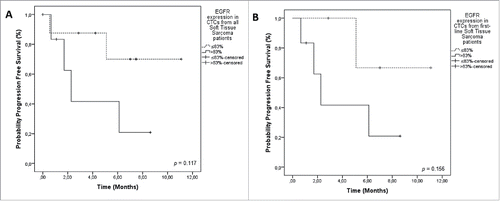
Figure 2. Progression-Free Survival (PFS) in relation to EGFR staining on CTCs from sarcoma patients. A) PFS of all patients included. EGFR expression in STS patients (> 83% = positive EGFR staining on CTCs 2.2 months); ≤ 83% = negative EGFR staining on CTCs (NR) (p = 0.117). B) PFS including only patients treated in first line. EGFR + CTCs was 2.2 months versus NR, for EGFR- CTCs (p = 0.156). Notes: Dotted line: patients without expression of EGFR. Continuous line: patients with expression of EGFR. NR = not reached.