Figures & data
Figure 1. MNK inhibits HIF-1α activity. (A). PC3 cells stably transfected with pGL4.27-HRE-LUC were pretreated with different concentrations of MNK under hypoxia (1% O2) for 5 h, the luciferase activity was examined. (B). Q-PCR analysis of indicated HIF-1α target genes in LNCaP cells treated with 60 μM MNK under hypoxia for 5 h. Columns represent fold changes. Error bars indicate mean ± SD. ∗, P < 0.05; ∗∗∗, P < 0.001.
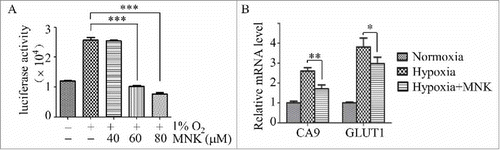
Figure 2. MNK decreases HIF-1α protein. PC3 cells (A) and LNCaP cells (B) were treated with the indicated concentrations of MNK under hypoxia (left) for 5 h or cobalt chloride for 6 h (right). Cell lysates were subjected to immunoblot assays for HIF-1α and β-tubulin. The experiments were repeated three times.
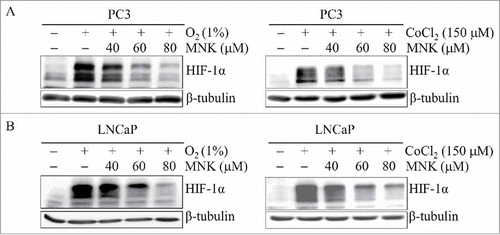
Figure 3. MNK-induced HIF-1α protein reduction is independent on protein degradation and leukotriene receptor. PC3 cells were treated with MNK for 6 h in the presence or absence of MG132 (A) or CQ (B) in the presence of 150 μM CoCl2. The indicated proteins were examined by western blot. (C). PC3 cells were treated with different concentrations of zafirlukast and pranlukast for 6 h in the presence of 150 μM CoCl2. Cell lysates were subjected to immunoblot assays. β-tubulin was used as loading control. All experiments were repeated three times. Za, zafirlukast; Pra, pralukast.
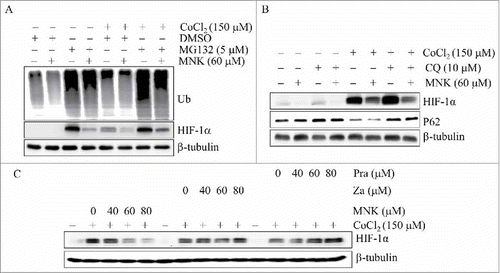
Figure 4. PERK is involved in MNK-induced reduction of HIF-1α (A). PC3 cells were treated with 80 μM MNK in the presence of 150 μM CoCl2 for 6 h in the presence or absence of GSK2606414. The indicated proteins were examined by western blot. β-tubulin was used as loading control. The experiments were repeated three times.
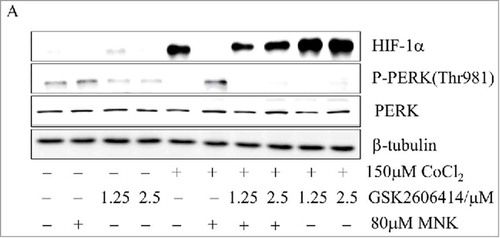
Figure 5. MNK induces phosphorylation of eIF-2α and inhibits translation of HIF-1α PC3 or LNCaP cells were treated with indicated concentrations of MNK in the presence or absence of 150 μM CoCl2 or under hypoxia (1% O2) for 6 h, cell lysates were prepared and the indicated proteins were examined by western blot (A-C). (D). PC3 cells transfected with vector and EIF2S1-S51A plasmid then treated with 60 μM MNK under hypoxia (1% O2) for 6 h. Cell lysates were subjected to immunoblot assays. β-tubulin was used as loading control. (E). MNK leads to the phosphorylation of eIF-2α (Ser51), which in turn results in the inhibition of protein translation including HIF-1α.
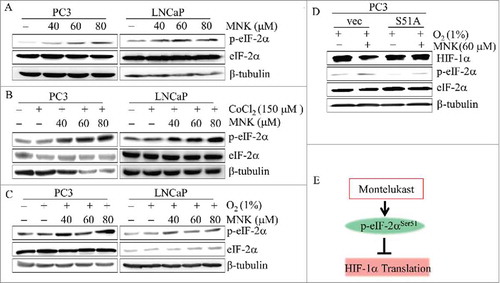
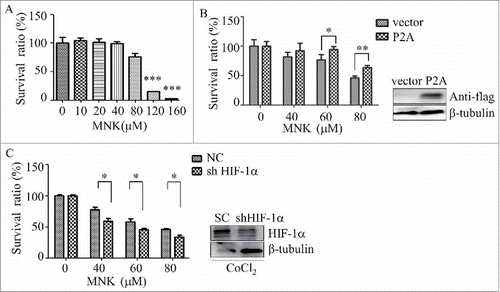
Figure 6. HIF-1α participates in MNK-induced proliferation inhibition of PC3 cells (A). PC3 cells were incubated with indicated concentrations of MNK for 12 h, and then cell proliferation was evaluated by CCK-8 assay. (B). PC3 cells transfected with HIF-1α-P2A were treated with MNK for 12 h and cell proliferation was evaluated by CCK-8 assay. (C). HIF-1α gene expression was reduced by short hairpin RNA (shRNA), and then the cells were treated with MNK in the presence of CoCl2. Then the cell proliferation was evaluated by CCK-8 assay. ∗, P < 0.05; ∗∗, P < 0.01. All experiments were repeated three times.