Figures & data
Figure 1. The S47 variant of p53 is impaired for transcription-independent apoptosis and mitochondrial localization of p53 in response to cisplatin.
(A) WT and S47 MEFs were treated with 10 μM CDDP for 0, 6, 24 hours and subjected to analysis by western blot using antibodies for the proteins indicated. HSP90 is included as a loading control. The data depicted are representative of three independent experiments from several independent MEF cultures of each genotype. (B,C) Western blot analysis of whole cell lysate (WCL) (B) and lysate from purified mitochondria (Mito) versus cytosolic fraction (Cyto) (C) isolated from WT and S47 MEFs untreated or treated with 10 μM CDDP for 12 hours. Lysates were probed for the mitochondrial proteins HSPA9 (GRP75) and cytochrome c, and the nuclear/cytosolic protein PCNA as an assessment of purity.
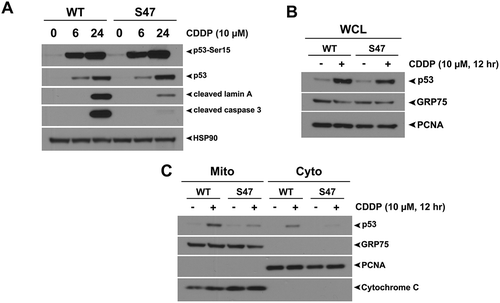
Figure 2. Equivalent transcription-independent apoptosis and mitochondrial localization of WT p53 and S47 in response to camptothecin.
(A) WT and S47 MEFs were treated with 5 μM camptothecin (CPT) for 0, 6, 24 hours and subjected to analysis by western blot using antibodies for the proteins indicated. HSP90 is included as a loading control. The data depicted are representative of three independent experiments from several independent MEF cultures of each genotype. (B,C) Western blot analysis of whole cell lysate (WCL) (B) and lysate from purified mitochondria (Mito) versus cytosolic fraction (Cyto) (C) isolated from WT and S47 MEFs untreated or treated with 5 μM CPT for 12 hours. Lysates were probed for the mitochondrial proteins HSPA9 (GRP75) and cytochrome c, and the nuclear/cytosolic protein PCNA as an assessment of purity.
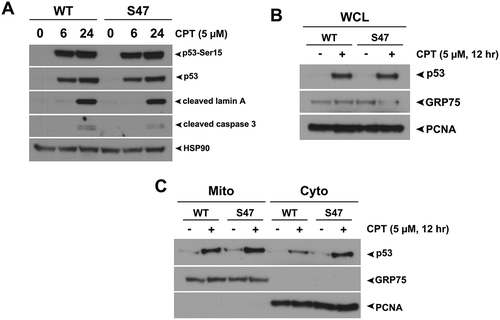
Figure 3. Proximity ligation assays (PLA) reveal a cisplatin-specific defect for S47 in mitochondrial localization.
(A, B) An in-situ proximity ligation assay (PLA) was performed in WT and S47 MEF cells treated with either 10 μM cisplatin (CDDP) (A) or 5 μM camptothecin (CPT) (B) for 12 hours. Each red dot represents an interaction between endogenous p53 and TOMM20 proteins (scale bar, 25 μm). Cells stained with p53 antibody alone were used as a negative control. DAPI nuclear staining is shown in blue. The white boxed image is magnified in the panel to the right. S47 cells treated with CDDP show a significantly lower number of PLA-positive dots compared to WT cells, indicating a defect in S47 trafficking to the mitochondria.(C) Quantification of (A and B), measured as the average number of PLA signals per nuclei. Data were quantitated by counting the number of cells in five random fields of view per experimental group.
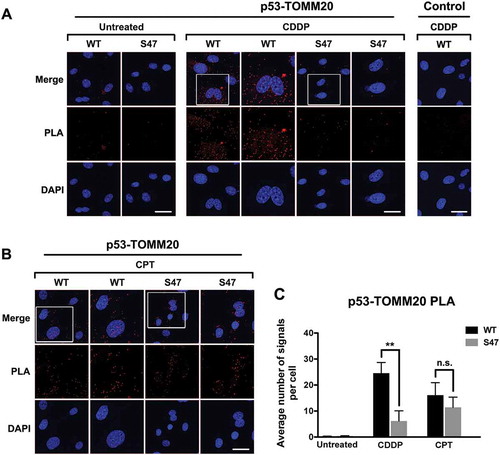
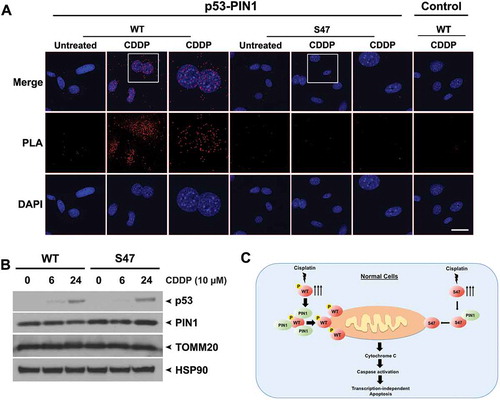
Figure 4. Decreased binding of S47 to PIN1 following cisplatin treatment.
(A) An in-situ proximity ligation assay (PLA) was performed in WT and S47 MEFs untreated or treated with 10 μM cisplatin (CDDP) for 12 hours. Each red dot represents an interaction between endogenous p53 and PIN1 proteins (scale bar, 25 μm). Cells stained with p53 antibody alone were used as a negative control. DAPI nuclear staining is shown in blue. The white boxed image is magnified in the panel to the right.(B) Western blot analysis of PIN1 and TOMM20 protein levels in WT and S47 MEFs treated with 10 μM CDDP for 0, 6, 24 hours. HSP90 is included as a loading control.(C) Proposed model highlighting the defect in mitochondrial trafficking and cell death in S47 cells treated with cisplatin.