Figures & data
Figure 1. Mechanism of action of temozolomide. Temozolomide is stable at acidic pH. At physiological pH, it is chemically converted to 5-(3-methyltriazol-1-yl)imidazole-4-carboxamide (MTIC, active compound. MTIC is further hydrolyzed to 5-amino-imidazole-4-methyl Amide (AIC) and methyldiazolium. Methyldiazo ions react with DNA and release its methyl group.
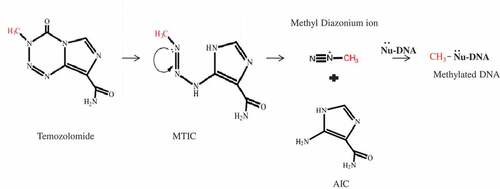
Figure 2. Adduct formation as a result of the reaction of DNA and temozolomide. A) N7 methylguanine (N7MG): The N7 position of guanine is methylated by temozolomide. The 70% adduct formed in the cells under temozolomide exposure was N7MG. B) N3 methyl adenine (N3MA): The N3 position of adenine is methylated by temozolomide. The 10% adduct formed in the cells under temozolomide exposure was N3MA. C) O6 methylguanine (O6MG): The O6 position of adenine is methylated by temozolomide. The 5% adduct formed in the cells upon exposure to temozolomide is O6MG.
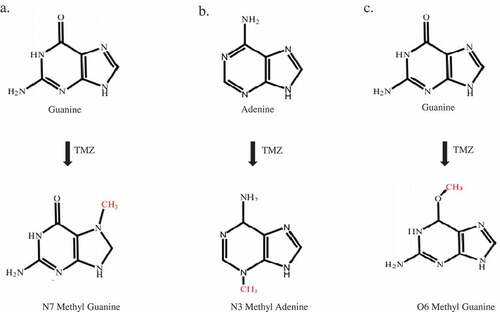
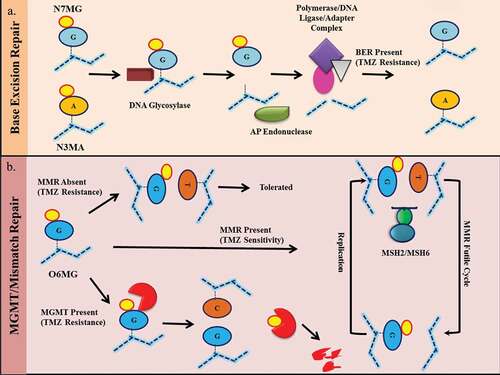
Figure 3. Resistance mechanism of temozolomide. A) N7MG and N3MA induced base excision repair (BER) pathways leading to recognition of modified bases by DNA glycosylation enzymes followed by base excision by AP endonuclease. This results in the recruitment of complexes containing DNA polymerase, DNA ligase, and adaptor molecules. This complex replaces the damaged site with the correct foundation. An effective BER results in resistance to temozolomide. B) O6MG on DNA results in an O6MG-thymidine mismatch. In the presence of active mismatch repair (MMR), the incorporated thymine was removed from the undamaged strand and incorporated again during the next replication cycle. This results in an ineffective loop of MMR which results in cytotoxicity. In the absence of an active MMR mechanism, the O6MG-thymidine mismatch is tolerated and cells survive. In the presence of MGMT, the O6MG is repaired, resulting in resistance.
Box 1. Red queen race conversation from Alice in the wonderland by Lewis Carroll.
Box 2. Methods for MGMT measurement.
