Figures & data
Table 1. Results of NGS examination
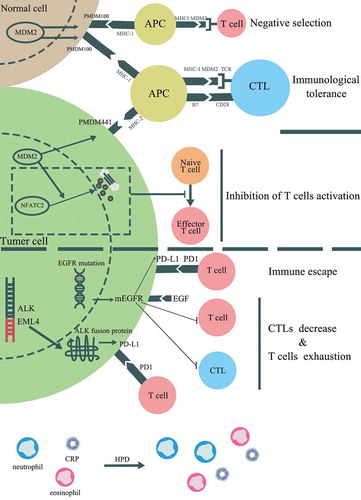
Figure 1. Clinical and histological data of the patient from our center. (a) The timeline of treatments for a patient with advanced esophageal squamous carcinoma. (b) The PD-L1 expression in tissue sample by IHC using 22C3 (X200)
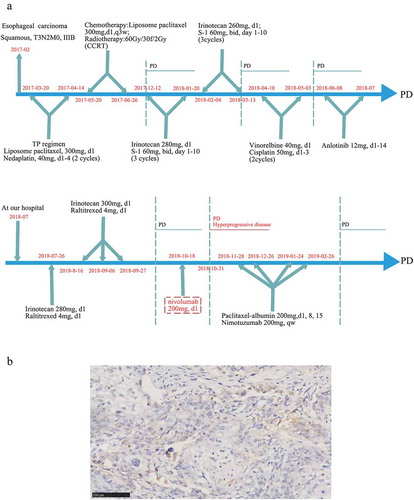
Figure 2. CT scan and tumor markers of the patient experienced HPD after nivolumab treatment. (a) The CT scan before and after nivolumab treatment. In this case, HPD was defined as a disease condition after anti-PD1/PD-L1 treatment which has a short TTF (less than 1 month) and a more than 50% increase than baseline in the size of the lesion. (b) The alterations in tumor markers during nivolumab treatment
Table 2. The HPD incidences among patients received ICIs
Figure 3. Potential mechanisms associated with HPD induced by immunotherapy. In addition to PI3K/AKT pathway, several genetic alterations and clinical factors have been stated to be involved in HPD, which including mouse double minute 2 (MDM2) or MDM4 amplification, alteration of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). The potential cell signaling pathways contributed to HPD were displayed