Figures & data
Figure 1. FDA-approved drugs in advanced ovarian cancer
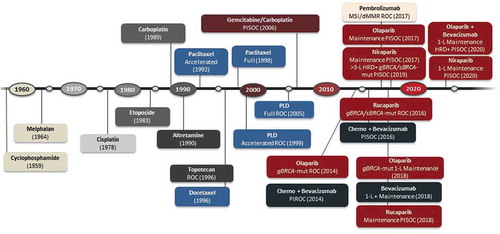
Table 1. Pivotal trials and FDA approvals of PARP and VEGF inhibitors in advanced ovarian cancer
Table 2. Pivotal trial results for front-line maintenance therapy in patients with newly diagnosed, advanced ovarian cancer after response to platinum-based therapy
Table 3. Single-agent treatment with PD-1/PD-L1 immune checkpoint inhibitors in patients with advanced ovarian cancer
Table 4. Investigational combination regimens in patients with advanced ovarian cancer
Table 5. Targeted agents in development for the treatment of patients with advanced ovarian cancer
Table 6. Clinical trials evaluating emerging agents for the treatment of patients with advanced ovarian cancer
