Figures & data
Figure 1. LLY17 and LLL12B inhibited IL-6 induced phosphorylation of STAT3 in human medulloblastoma cells. D283, D425, UW288, and UW426 human medulloblastoma cells were seeded and cultured overnight. Cells were pre-treated with LLY17 (1 µM, 2.5 µM and 5 µM), LLL12B (0.5 µM, 1 µM, and 2.5 µM) or DMSO for 4 hours in 0% FBS, then stimulated by 50 ng/ml IL-6 for additional 30 minutes and harvested for Western blot. Western blot analysis of P-STAT3 (Y705) and STAT3 in LLY17 (a) and LLL12B (b) treated cells. GAPDH was used as a protein loading control
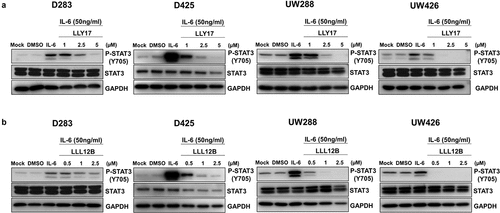
Figure 2. LLY17 or LLL12B and irradiation combination treatment significantly inhibited cell viability in human medulloblastoma cells. Cells were seeded and cultured overnight in 96-well plates. Non-irradiated cells or irradiated (4 Gy) cells were treated with LLY17, LLL12B, or DMSO for 72 hours. The combination effects of irradiation and LLY17 (a) or LLL12B (b) on cell viability were determined by MTT assay. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001
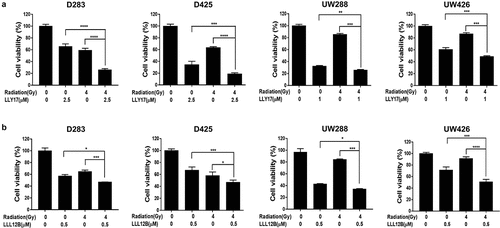
Figure 3. The inhibitory effects of LLY17 or LLL12B combined with irradiation on cell migration in human medulloblastoma cells. Wound healing assay was performed to determine the migratory ability of irradiated and LLY17 or LLL12B treated UW288 and UW426 cells. Cells with combination treatment were compared to cells with single treatment or with DMSO control. Representative cell images showing the scratch at 0 h and 24 h after non-irradiation or irradiation and LLY17 (1 µM) or DMSO treatment in UW288 cells (a) and UW426 cells (b). Quantification of the migration area is shown in the right panel. Representative cell images showing the scratch at 0 h and 24 h after non-irradiation or irradiation and LLL12B (0.5 µM) or DMSO treatment in UW288 cells (c) and UW426 cells (d). Quantification of the migration area is shown in the right panel. Experiments were performed in triplicate and data are presented as means ± SD. **P < .01, ***P < .001, ****P < .0001
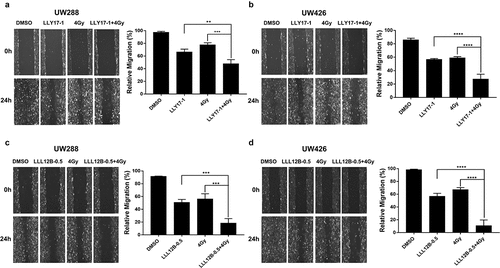
Figure 4. The inhibitory effects of LLY17 or LLL12B combined with irradiation on cell invasion and EMT in human medulloblastoma cells. Invasion assay was performed to determine the invasion ability of irradiated and LLY17 or LLL12B treated human medulloblastoma cells. Cells were treated with DMSO, LLY17 or LLL12B alone, with or without irradiation (4 Gy) for 24 h. Representative images of D283 (a), D425 (b), UW288 (c), and UW426 (d) cells are shown. Quantification of cell invasion is shown in the bottom panel. Human medulloblastoma cells were treated with LLY17 (e) or LLL12B (f) alone, with or without irradiation (4 Gy) overnight and the expression levels of P-STAT3 (Y705), STAT3, N-cadherin, and SLUG were determined by Western Blot. GAPDH was used as a protein loading control. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, ***P < .001
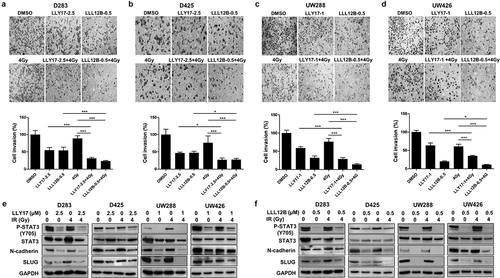
Figure 5. LLY17 or LLL12B combined with irradiation enhanced the inhibition of tumorsphere formation in human medulloblastoma cells. D283 and D425 cells were dissociated and plated in ultra-low attachment 6-wells at 10,000 cells/ml. Non-irradiated or irradiated (4 Gy) cells were treated with LLY17, LLL12B, or DMSO for 10 days. Cells with combination treatment were compared to cells with single treatment or with DMSO control. (a) Representative images of tumorspheres following LLY17 treatment without or with irradiation in D283 and D425 cells. (b) Quantification of tumorsphere numbers in D283 and D425 cells following LLY17 treatment without or with irradiation. (c) Representative images of tumorspheres following LLL12B treatment without or with irradiation in D283 and D425 cells. (d) Quantification of tumorsphere numbers in D283 and D425 cells following LLL12B treatment without or with irradiation. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001
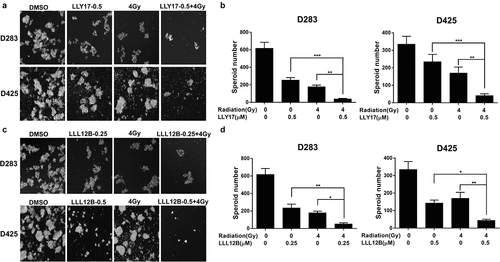
Figure 6. LLY17 or LLL12B combined with irradiation induced apoptosis in human medulloblastoma cells. Cells were treated with DMSO, LLY17, or LLL12B alone, with or without irradiation (4 Gy) overnight and subjected to the Annexin V apoptosis assay using flow cytometry. Representative results of the assay in D283 (a), D425 (b), UW288 (c) and UW426 (d) cells are shown. The percentage of apoptotic cells (early and late apoptosis) was quantified and shown in the right panel. Experiments were performed in triplicate and data are presented as means ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001
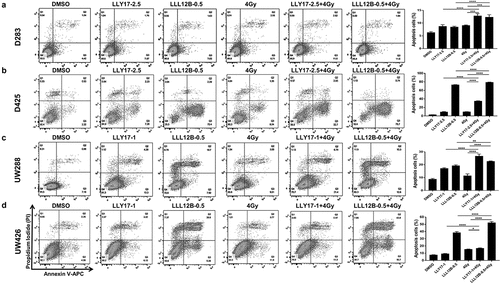
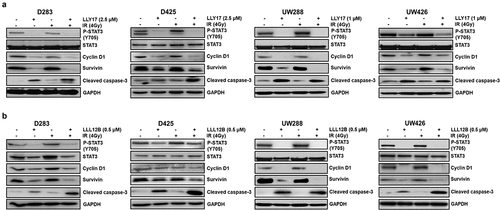
Figure 7. LLY17 or LLL12B combined with irradiation inhibited STAT3 targets and induced cell apoptosis protein in human medulloblastoma cells. Non-irradiated or irradiated (4 Gy) human medulloblastoma D283, D425, UW288, and UW426 cells were treated with LLY17 (a), LLL12B (b) or DMSO overnight. Cells were harvested and analyzed by Western blot. The expression levels of P-STAT3 (Y705), STAT3, Cyclin D1, Survivin and cleaved Caspase-3 were determined. GAPDH was used as a protein loading control