Figures & data
Figure 1. CLIP-170 and Tctex-1, but not dynein, remain at attached kinetochores without mDia3 and EB1. (A–C) Indirect immunofluorescence staining of DNA (DAPI staining) and ACA, along with EB1 (A), CLIP-170 (B), Dynein (DIC in C), and Tctex-1 (C) in control and mDia3 knockdown metaphase cells as indicated (72 hr post-transfection). Cells were treated with nocodazole for 4 hrs and then released into MG132 for 1 hr prior to fixation. Bar, 5 μm. (D) Quantification of the relative intensities (per kinetochore) at aligned kinetochores of EB1/ACA, CLIP-170/ACA, DIC/ACA, and Tctex-1/ACA in metaphase cells (mean with 95% confidence interval, P < 0.0001 as indicated by *** based on more than 30 kinetochores from at least 5 cells).
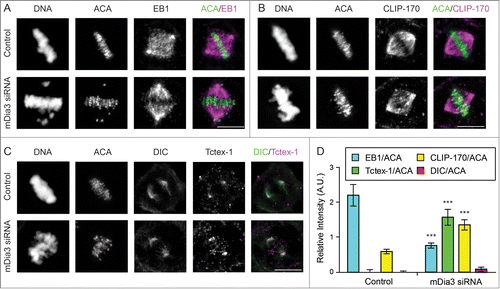
Figure 2. The dynein light chain Tctex-1 associates with unattached kinetochores. (A) Immunofluorescence detection of ACA, Tctex-1, and DNA (DAPI staining) in mitotic cells. In merged panels: ACA – green, Tctex-1 – red, and DNA – blue. Insets: colocalization of Tctex-1 and ACA at a pair of sister kinetochores. Arrows point to spindle poles and arrow heads point to unattached kinetochore on a polar chromosome. Bar, 5 μm. (B) Left panel: N-SIM Super-resolution images showing Hec1 (green) and Tctex1 (magenta). Right panel: graph showing a representative linescan of the fluorescent intensity of a pair of sister kinetochores that is highlighted in left panel (inset).
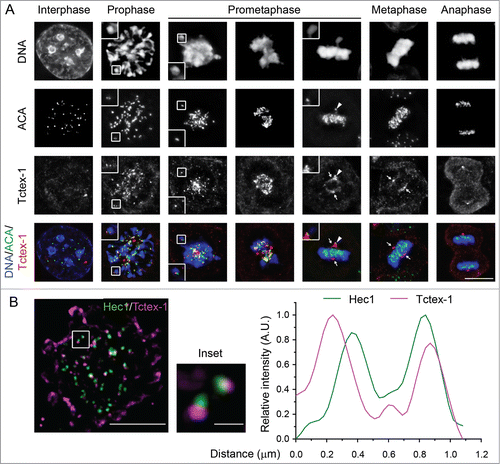
Figure 3. Tctex-1 is essential for accurate chromosome segregation. (A) Immunoblotting of cell lysates 48 hr posttransfection with control or Tctex-1 shRNA plasmids as indicated. (B) Immunofluorescence staining of ACA and Tctex-1 in mitotic cells 48 hr posttransfected with control or Tctex-1 shRNA plasmids, as well as the wild-type Flag-Tctex-1 expression vector, as indicated. For Tctex-1 shRNA + Flag-Tctex-1 cells, Tctex-1 was stained with anti-Flag antibody. Bar, 5 μm. (C) Time spent from nuclear envelope breakdown to metaphase (or pseudometaphase) (green) and from metaphase (or pseudometaphase) to anaphase onset (magenta) of cells (expressing H2B-EYFP) transiently transfected with control or Tctex-1 shRNA plasmids, as well as the wild-type Flag-Tctex-1 vector (72 hr post-transfection). Each vertical bar represents a single cell. The transfected cells were identified by HcRed, which are coexpressed by the shRNA plasmid, before live cell imaging. Pseudometaphase is designated as when the majority of chromosomes are aligned with an obvious metaphase plate and a few polar chromosomes. (D) Stills of live-cell imaging showing cells transfected with control or Tctex-1 shRNA plasmids, as well as the wild-type Flag-Tctex-1 expression vector, as indicated, during unperturbed mitoses. The transfected cell was identified by cytosolic red signals from the co-expression of HcRed as showed in the last still image. Bar, 5 μm.
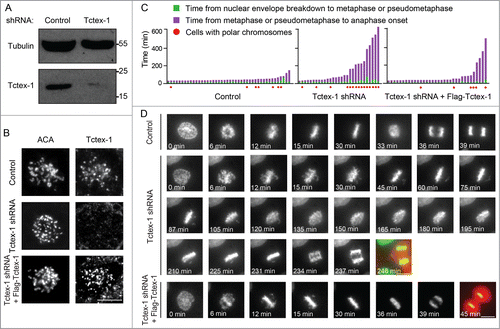
Figure 4. The mitotic role of Tctex-1 does not depend on its interaction with dynein. (A) Immunoprecipitates from mitotic cell extracts with DIC antibody and then probed with DIC and Flag antibodies as indicated. Cells were transfected with Flag tagged wild-type Tctex-1, Tctex-1T94A or Tctex-1-T94E. (B) Time spent from nuclear envelope breakdown to metaphase (or pseudometaphase) (green) and from metaphase (or pseudometaphase) to anaphase onset (magenta) of cells (expressing H2B-EYFP) transiently transfected with Tctex-1 shRNA plasmids and expression vectors for the nonphosphorylatable Tctex-1T94A or the phosphomimetic Tctex-1T94E variants (72 hr post-transfection). Each vertical bar represents a single cell. (C) Stills of live-cell imaging showing cells transfected with Tctex-1 shRNA plasmids and expression vectors for the nonphosphorylatable Tctex-1T94A or the phosphomimetic Tctex-1T94E variants, as indicated, during unperturbed mitoses. Bar, 5 μm. (D) Distribution of total time spent in mitosis (from nuclear envelope breakdown to anaphase onset) in cells transfected with shRNAs and expression vectors as indicated. Whisker-Tukey boxes span 25-75 percentile, while center bar denotes median and “+” marks mean. (E) Stills of live-cell imaging showing a control cell and cells transfected with Tctex-1 shRNA plasmids at the anaphase onset. Arrows indicate missegregated polar chromosomes and arrow heads indicate chromosome bridges. Bar, 5 μm. (F) The percentage of mitotic cells with normal segregation (blue), missegregated polar chromosomes (green), chromosome bridges (yellow), and both (red) at the anaphase onset were analyzed in cells transfected with shRNAs and expression vectors as indicated. Mean with 95% confidence interval were plotted with chi-square test used to compute the p-value.
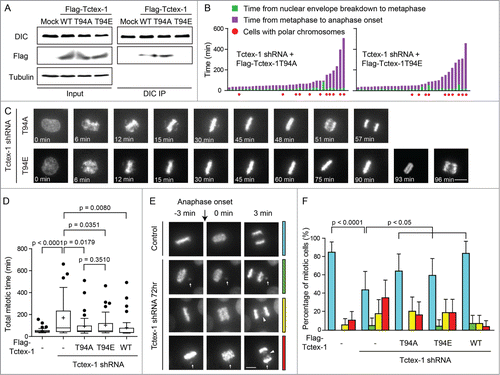
Figure 5. Tctex-1 depletion does not affect the association of the mitotic checkpoint proteins at unattached kinetochore and their release from attached kinetochores. (A and B) Immunofluorescence detection of DNA, ACA, BubR1 (A) and Mad1 (B) in prometaphase and metaphase cells transfected with control or Tctex-1 shRNA plasmids as indicated. Bar, 5 μm. (C) Quantification of the normalized relative kinetochore intensities (per kinetochore) of Mad1/ACA in mitotic cells. Whisker-Tukey boxplots span 25–75 percentile, while center bar denotes median and “+” marks mean. Quantifications were based on 30 kinetochores (control unaligned), 30 kinetochores (control aligned), 37 kinetochores (Tctex-1 shRNA unaligned) and 30 kinetochores (Tctex-1 shRNA aligned) from multiple cells.
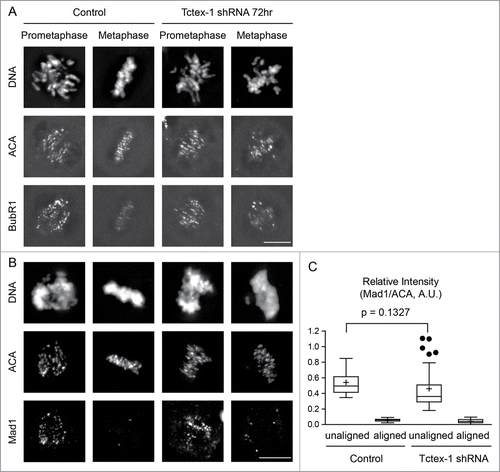
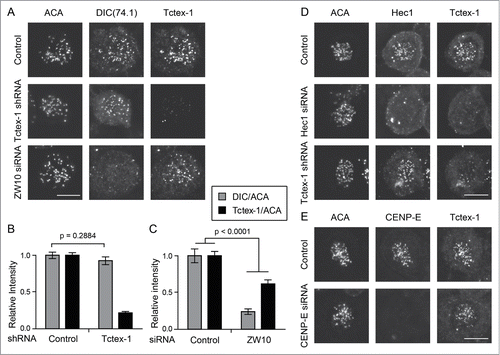
Figure 6. Tctex-1 kinetochore localization is dynein independent. (A) Immunofluorescence detection of ACA, dynein (DIC), and Tctex-1 in nocodazole treated (3 hrs) mitotic cells transfected with Tctex-1 shRNA plasmids or ZW10 siRNA as indicated. Bar, 5 μm. (B and C) Quantification of the normalized relative kinetochore intensities (per kinetochore) of DIC/ACA and Tctex-1/ACA (mean ± SEM) in mitotic cells. In (B), 63 (control) and 57 (Tctex-1 shRNA) kinetochores from multiple cells were measured. In (C), 83 (control) and 41 (ZW10 siRNA) kinetochores from multiple cells were measured. (D and E) Immunofluorescence detection of ACA, Hec1 (A) or CENP-E (B), and Tctex-1 in mitotic cells transfected with Hec1 (A) or CENP-E (B) siRNA. Bar, 5 μm.