Figures & data
Figure 1. The CAK participates in both CDK-cyclin activation and transcription initiation. CAK has been shown to be required for the activation of CDK1-cyclin B at mitosis and is also recruited into the TFIIH general transcription factor via its XPD subunit and, as part of this complex, it triggers the initiation of RNA polymerase II transcription through phosphorylation of the carboxy terminal domain of its largest subunit. Degradation of XPD, likely triggered after phosphorylation by CDK1-cyclin B, is proposed to be responsible for mitotic silencing of transcription.
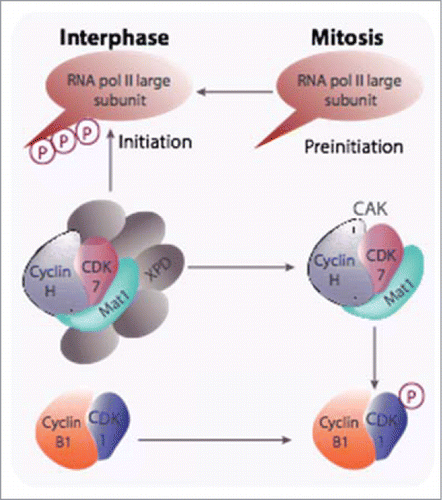
Figure 2. E-type cyclins together with cyclin A2 are involved in the tight linkage between the nuclear and centrosomal cycles. E-type cyclins facilitate MCMs loading through a physical interaction with these proteins as well as with CDT1Citation35. Similarly to chromosomes, centrosomes must be duplicated, and this takes place at the onset of S phase to allow the faithfully duplicated organelles to move to the poles of the duplicating cell and then, to be distributed to the daughter cells. Both cyclins E and cyclin A2 have also been implicated in this phenomenon (Pascreau et al, 2011).
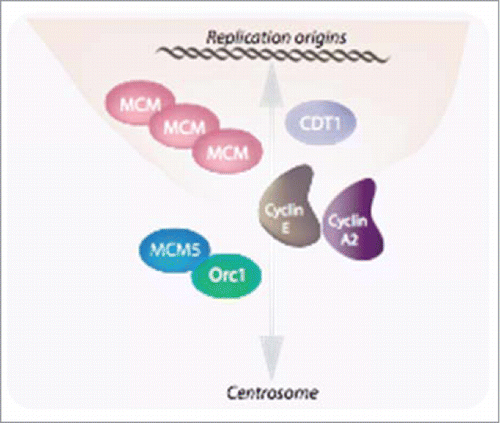
Figure 3. Cyclin E is involved in cell fate determination during early neurogenesis in Drosophila. Through asymmetric divisions, progenitors, or neuroblasts, give rise to both neurons and glial cells. Whereas in the thoracic segments of the embryonic nervous system neuroblasts divide first asymmetrically, giving rise to both a glial and a neuronal lineage, abdominal neuroblasts divide once symmetrically into 2 glial cells. Cyclin E is required for maintaining the transcription factor Prospero in a cortical localization in neuronal precursors and is inhibited to do so by the Abdominal-A and Abdominal-B gene products (Abd-A, Abd-B)
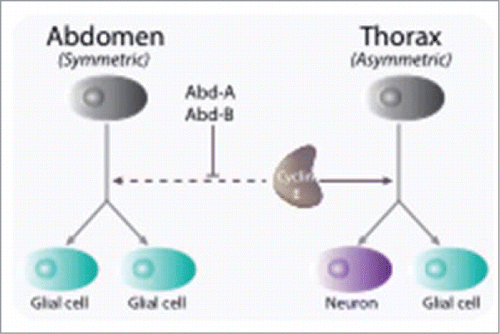
Figure 4. Cyclin D1 plays a central role in transcription. A few examples are given in this figure. Aside from its role in the cell cycle-dependent phosphorylation of pRB, Cyclin D1 harbors both CDK-dependent (NRF-1) and CDK-independent (C/EBPβ, ER) roles in the transcriptional control of genes required for the metabolism.
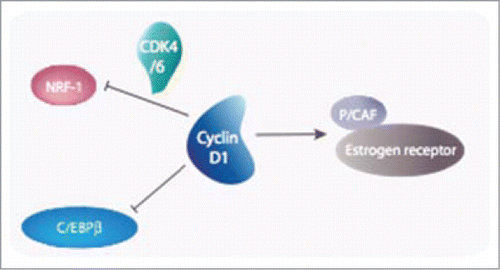
Table 1 Some of cyclin/CDK relevant substrates for cell migration/actin cytoskeleton regulation
Figure 5. Cyclins and CKIs regulate cell motility. (A) Cyclins and CKIs target RhoA-ROCK pathway: RhoA, stimulated by its GEF activates ROCK , that in return stimulates LIMK. Cyclins and CKI regulate the pathway at multiple levels. Phosphorylated cytoplasmic p27 interferes between RhoA and its GEF and also inhibit stathmin. Cyclin D1 represses ROCK and Thrombospondin gene transcription and when bound to p27, it interferes with RhoA activation. Furthermore, Cyclin D1 indirectly stabilizes p27 via the regulation of kinases involved in its degradation or its cytoplasmic localization. Cyclin A2, independently of CDKs binding and via direct interaction with RhoA and RhoC, regulates their activity. In contrast to p27, cyclin A2 stimulation RhoA GEFs. How cyclin A2 interferes with RhoC activation during EMT and whether it requires CDKs binding is still unclear. p21 and p57 target kinases downstream of RhoA. While cytoplasmic p21 directly inhibit ROCK activity, p57 sequesters LIMK in the nucleus, away from its substrates. (B) Cyclin B1-CDK1 is not only involved in phosphorylation of substrates involved in cell cycle progression, but also in the phosphorylation of a plethora of other targets including molecules intervening in cell motility.
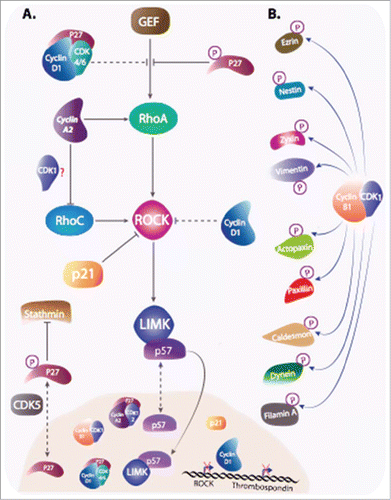
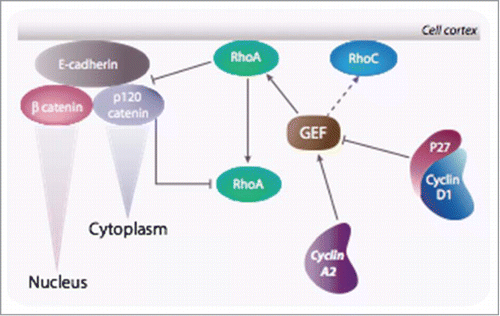
Figure 6. Cyclin A2 controls EMT via a reciprocal regulation of RhoA and RhoC: Cyclin A2 potentiates the loading activity of Rho GEF toward RhoA at the expense of RhoC. Since RhoA is required for the stability of the adherens junction, ablation of cyclin A2 leads to its inhibition and, as a consequence, in the disruption of cell-cell contacts. Accordingly, RhoC activity is increased and contributes further in the instability of the junction. As a result, p120 catenin translocates to the cytoplasm where it forms an inactivating complex with RhoA, and β catenin enters the nucleus where it participates in the transcription of genes required for the EMT. In cells overexpressing cyclin D1 this phenomenon is exacerbated.