Figures & data
Figure 1. Gprc5a-ko mice are susceptible to endotoxin-induced pulmonary edema and injury. (A) Body weight of Gprc5a-ko (5a-KO) and wild-type (WT) mice (n = 3) treated with endotoxin over 48 h period after treatment. Weights were normalized to baseline weight in each animal. (B) Lung water measurements (wet:dry tissue weight) in control and endotoxin-treated mice over 48 h period after treatment. Ratio of wet-dry weights are normalized to the baseline values for each point (n = 3). (C) Photomicrographs are representative hematoxylin and eosin (H&E) stained sections of lung tissue from wild-type and Gprc5a-ko mice obtained before endotoxin (0h) and at 6 h, 24 h, and 48 h after endotoxin administration. (D) Inflammation score (IS) was used as a semi-quantitative assessment for pulmonary edema and injury.
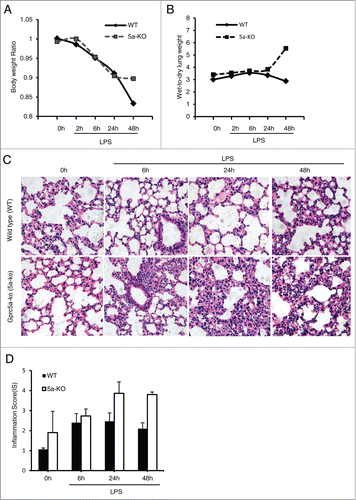
Figure 2. Lungs from Gprc5a-ko mouse produce increased levels of proinflammatory cytokines and chemokines after LPS treatment. (A) Images are representative of the RT-PCR analysis for mRNA of proinflammatory cytokines at each time point. (B-D) Average mRNA levels for IL-1β (B), TNFα (C) and KC (D) at each point are shown. (E) The average of mRNA levels of IL-1β, TNFα, and KC from wild-type and Gprc5a-ko mouse lungs at 48 h after endotoxin treatment. (F-H) Protein levels of cytokines, IL-1β (F), TNFα (G) and IL-6 (H), in lung tissues were measured by ELISA after LPS treatment.
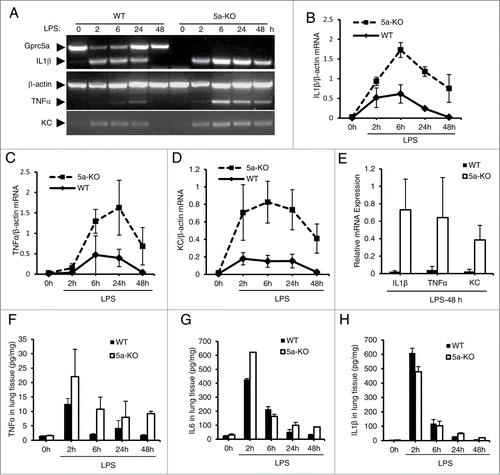
Figure 3. LPS induced potent and persistent activation of NF-κB in the bronchioalveolar epithelium of Gprc5a-ko mouse lungs. (A) Luminescence imaging from anesthetized mice of wild-type/NF-κB-luc and Gprc5a-ko/NF-κB-luc was performed with an ultrasensitive camera 48 h following endotoxin and luciferin administration. Tissue encircled in red is lung. (B) Images are of excised lung tissues from mice used above (A). NF-κB-dependent luminescence of lung tissue was assayed by imaging or luminometry. (C) RT-PCR analysis of mRNAs of NF-κB target genes, IκBα, MMP9, VEGF-C, Cyclin D1; times indicate tissue retrieval post-LPS treatment.
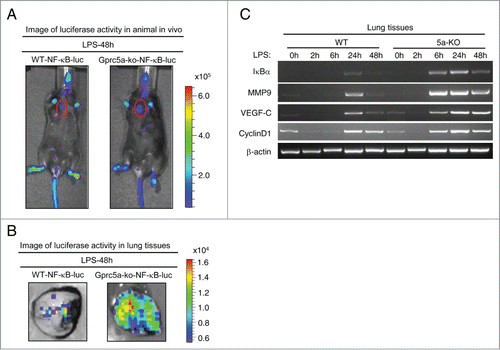
Figure 4. NF-κB signaling is selectively inhibited in the bronchioalveolar epithelium of Gprc5a-ko/SPC-SR-IκBα mouse lungs. (A) EMSA of NF-κB DNA-binding proteins in lung tissue nuclear extracts from Gprc5a-ko (5a-ko) and Gprc5a-ko/SPC-SR-IκBα(5a-ko/IκBα) mice treated with or without LPS. Specificity of NF-κB bindingconsensus site was analyzed by using (80 times) either wild-type or mutant oligonucleotides (cold) as competitors. Addition of anti-p65 antibodies to the EMSA reaction assessed the presence of p65 in the shifted complex by induction of a super-shifted complex (SS). (B) Representative RT-PCR analysis of NF-κB target genes MMP9, VEGF-C, Cyclin D1, from pulmonary tissues. Time points indicate retrieval of tissue post-LPS treatment. (C) Immunoblot analysis of NF-κB target gene products, MMP9, IκBα, Cox2, and Cyclin D1, from murine pulmonary homogenates.
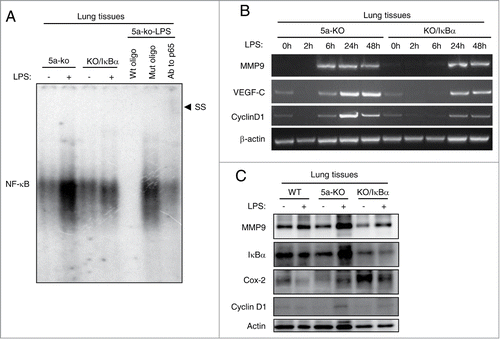
Figure 5. Gprc5a-ko/SPC-SR-IκBα mice are resistant to endotoxin-induced pulmonary edema and injury. (A) Body weight of Gprc5a-ko (5a-KO) and Gprc5a-ko/SPC-SR-IκBα (KO/IκBα) mice (n = 3) treated with endotoxin over 48 h period. Present data are weights normalized to baseline for each animal. (B) Lung water measurements (wet:dry tissue weight ratio) in mice treated with endotoxin and in control animals over 48 h period after treatment. Wet-dry weights are normalized to the baseline values (n = 3 for each time point). (C) H&E-stained sections of lung tissue from 5a-KO and 5a-KO/IκBα mice taken before endotoxin administration (0h) and at 6 h, 24 h, and 48 h after endotoxin administration. (D) Inflammation score (IS) was used as a semi-quantitative assessment for pulmonary edema and injury.
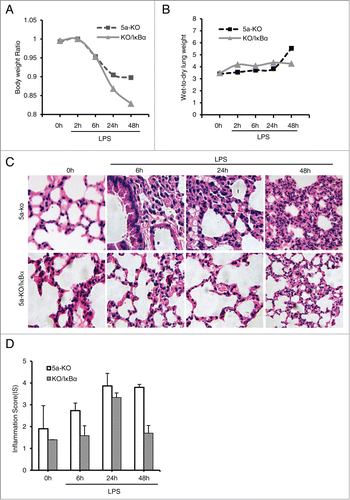
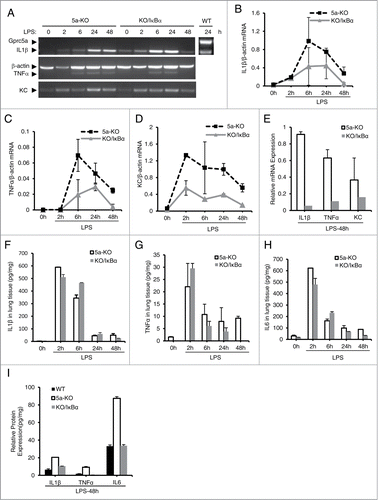
Figure 6. Production of proinflammatory cytokines and chemokines is reduced in Gprc5a-ko/SPC-SR-IκBα mouse lungs after endotoxin treatment. (A) Images are representative of RT-PCR analysis for proinflammatory cytokines at each point. The averages of mRNA, IL-1β (B), TNFα (C) and KC (D), at different time point were shown. (E) The average of mRNA expression levels for IL-1β, TNFα, and KC from wild-type and Gprc5a-ko mouse lungs at 48 h after endotoxin treatment. (F-I) Cytokine proteins, IL-1β (F), TNFα (G) and IL-6 (H), in lung tissues were measured after LPS treatment. (I) Protein levels for IL-1β, TNFα, and IL-6 from WT, KO and KO/IκBα mouse lungs 48 h after LPS treatment using ELISA.