Figures & data
Figure 1. p27Kip1-luciferase is stabilized by compounds from the Library of Pharmacologically Active Compounds and MG132. (A) Diagram of p27Kip1-luciferase used in our studies. Note that luciferase is attached to the C-terminus of full-length human p27Kip1. (B) HeLa cells were transfected with p27Kip1-PGL4 plasmids and incubated with either MG132 or DMSO (vehicle control). After 24 hours, cells were lysed using BriteLite and the steady-state levels of luciferase measured. Results are from one representative experiment performed in quadruplicate. (C) p27Kip1-luciferase is stabilized by compounds in the Library of Pharmacologically Active Compounds (LOPAC). HeLa cells were transfected with the p27Kip1-luciferase plasmid and incubated with compounds from a library of pharmacologically active compounds (LOPAC). Cells were then lysed after 24 hours and the steady-state levels of luciferase measured. The hit-cutoff based on the average +3 SD calculation method was 73.16%. Based on this cutoff, 21 compounds were designated as hits, i.e., a hit rate of 1.64% (21/1280), which is in the expected range of 1–2% when screening a library of pharmacologically active compounds (otherwise a hit rate of <1% is expected). Further, there were no false positives among the DMSO wells and therefore the assay is not prone to false positives. The plate-to-plate variability of the raw luminescence values was kept below <15% (12% CV for DMSO control from one plate to another, 8% CV for the MG132 controls).
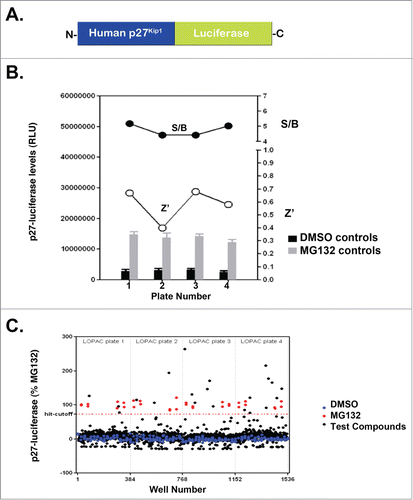
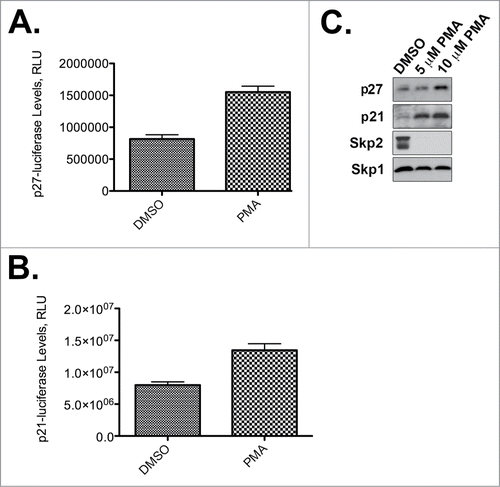
Figure 2. PMA stabilizes p27Kip1 and p21Cip1. (A) PMA stabilizes p27Kip1-luciferase. Results are from one representative experiment performed in quadruplicate. (B) PMA stabilizes p21Cip1-luciferase. Results are from one representative experiment performed in quadruplicate. (C) PMA stabilizes endogenous p21Cip1 and p27Kip1 and causes degradation of Skp2. HeLa cells were incubated with the indicated concentrations of PMA and processed for p21Cip1, p27Kip1, Skp2, or Skp1 immunoblotting. Skp1 is a loading control.