Figures & data
Figure 1. Dynamics of conversion of MEFs into neurons by exogenous BAM expression. (A) Experimental design. MEFs were plated at day -2, transduced with BAM+RT viruses at day -1 and cultured in the presence of DOX starting from day 0 until day 5–19. (B) Immunostaining with Tuj1 (yellow) and NF200 (red) antibodies in the targeted cells 5–19 d after viral transduction. (C) Same as B for neuronal markers Tuj1 (yellow) and MAP2 (red) antibodies. (D) Immunostaining with NF200 (red) and collagen-1 (green) antibodies of the targeted cells 19 d after viral transduction. Cell nuclei (DAPI) are shown in blue.
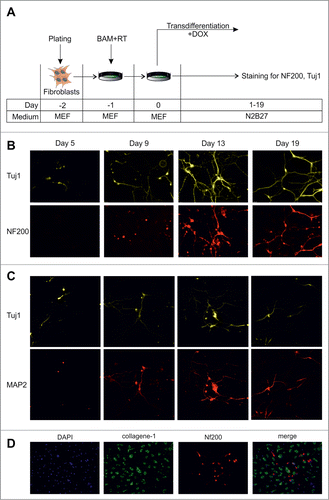
Figure 2. (See previous page) Direct conversion of MEFs into neuronal cells by BAM in the presence of cell division inhibitors. (A) BrdU incorporation in MEFs treated with cell division inhibitors aphidicolin or mimosine for 24 hr. (B) Number of MEFs survived after 2–10 d of culture in the presence of inhibitors. Aph – aphidicolin, Mim – mimosine, Ctr - untreated cells. X-axis indicates experimental time-frame starting from plating of MEFs (day -2) until day 10. Y-axis indicates the number of survived cells. The arrow shows the moment when medium was supplemented with cytostatics (day 0). The culture medium used at each time point of the experiment is shown in boxes under the plot. (C) Experimental design. MEFs were plated at day -2, transduced with BAM+RT viruses at day -1 and cultured in the presence of DOX and aphidicolin starting from day 0 until day 11. (D) Immunostaining with Tuj1 (yellow) and MAP2 (red) antibodies in the aphidicolin treated cells 5–11 d after viral transduction. (E) Tuj1/NF200 double-stained neurons generated from MEFs in the presence of aphidicolin 10 d after viral transduction. (F) Quantification of synapsin (Syn1) and Tuj1 expression (Log10 scale) in the aphidicolin treated cells 5–11 d after viral transduction. MEFs: control fibroblasts not transduced with viruses and cultured for 11 d in N2B27 media without aphidicolin *P < 0.05 in comparison to control MEFs.
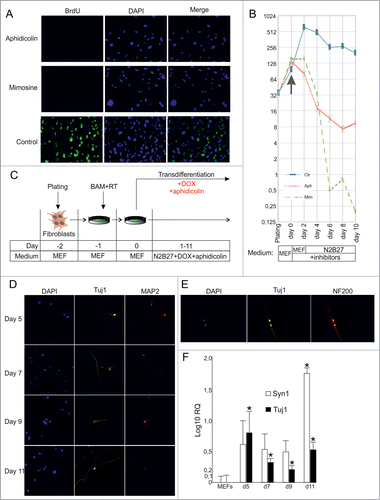
Figure 3. Direct conversion of MEFs to neurons without cell divisions. (A) Experimental design. MEFs were plated at day -2, transduced with BAM+RT viruses at day -1 and cultured in the presence of DOX from day 0 until day 11. Cells were treated with aphidicolin or mimosine starting from day 0 for 3 or 5 d. BrdU was first added 12 hours after cell division inhibitors (day 0.5) and was present in medium until the end of experiment. (B) Immunostaining with antibodies against Tuj1, NF200 and BrdU at day 11 of transdifferentiation, performed in the presence of aphidicolin from day 0 to day 5. Arrows show an example of cells treated with aphidicolin and positive for Tuj1 and NF200, but negative for BrdU. (C) Quantification of NF200+/Tuj1+/BrdU− cells obtained in the transdifferentiation experiments, performed in the presence of aphidicoline or mimosine during days 0–3 or 0–5 of transdifferentiation. BrdU− cells account for more than 70% of all NF200+/Tuj1+ neuronal-like cells. Aph – aphidicolin, Mim – mimosine. Number of experiments N = 3.
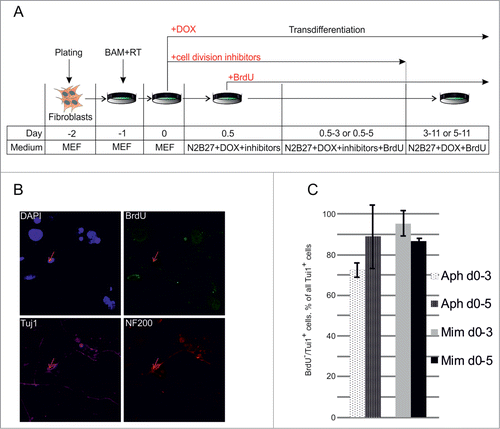
Figure 4. Generation of iPS cells from OG2 MEFs. (A) Morphology (PH) and GFP fluorescence of the iPS cells produced in the absence of aphidicolin at day 12 after viral transduction of OG2 MEFs, carrying GFP under the promoter of Oct4. (B) Morphology and GFP fluorescence of an iPS cell culture obtained from a single clone after 4 weeks expansion.
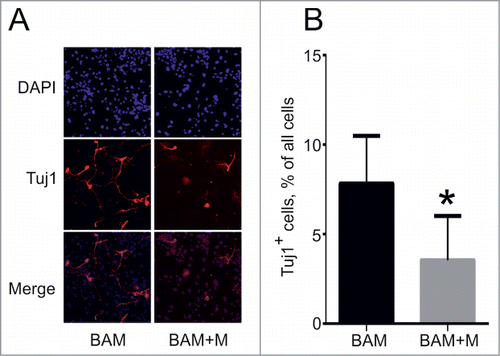
Figure 5. Effect of cMyc overexpression on direct conversion of MEFs into neuronal cells. (A) Phenotype of Tuj1+ neuronal cells (red) obtained from MEFs 18 d after transduction with BAM and BAM+M lentiviruses. (B) Percentage of Tuj1+ cells out of all cells obtained at day 18. BAM+M – MEFs transduced with BAM and cMyc viruses, BAM – MEFs transduced with BAM viruses only. Number of experiments for each condition: N = 6. Data are presented as means ± SEM (standard error of means). *P < 0.05